Figure 10.
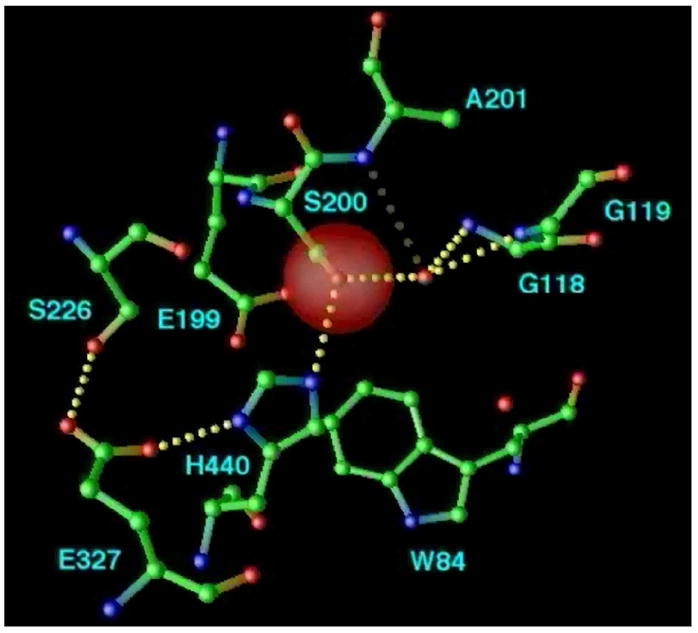
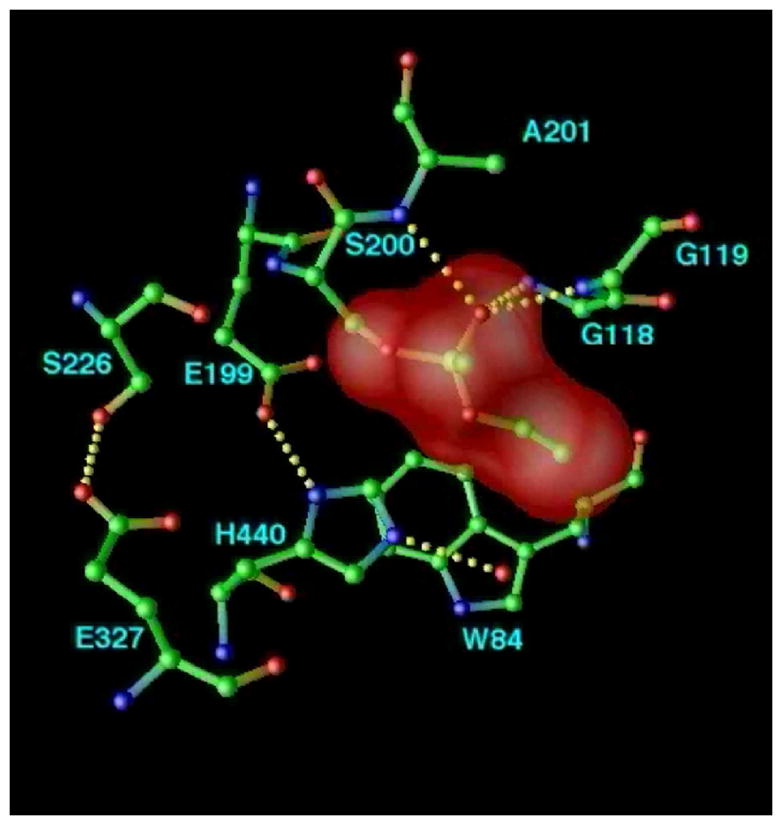
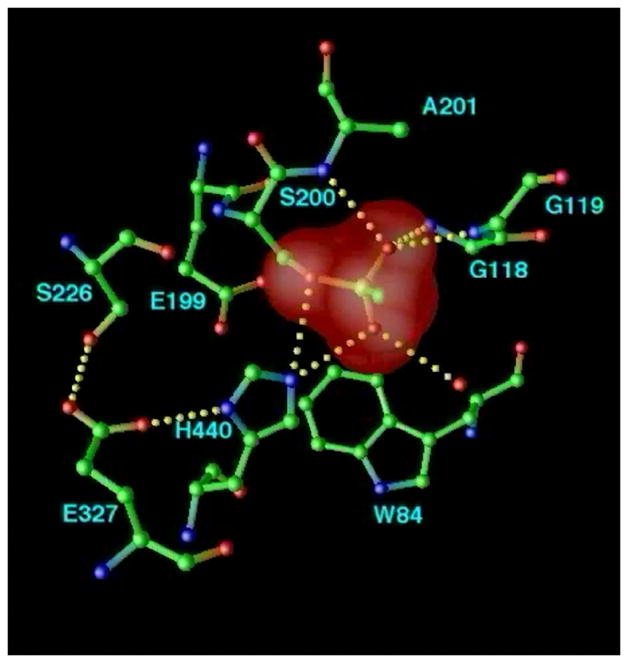
Structural kinetics of covalent modification of TcAChE by the nerve agent, VX, as monitored by X-ray crystallography. The active site of TcAChE is depicted with possible H-bonds involving the catalytic triad and the OP moiety (broken lines). (a) Native structure, showing the active site, including the catalytic triad (S200-H440-E327) and the oxyanion hole (-NH of G118, G119, and A201); (b) Pro-aged structure. Phosphonylation triggers a conformational change of H440 that disrupts the H-bond to G327; this may be caused either by steric crowding in the pentavalent phosphorus transition state, or by re-distribution of charge on the H440 imidazole during phosphonylation. It should be noted that E199 and a water molecule apparently stabilize the alternate conformation of H440. Subsequently, the H440 imidazole catalyzes either dealkylation (aging), or spontaneous reactivation; (c) Aged structure. For reaction of AChE with VX and with most phosphonates, aging predominates, and dealkylation results in movement of H440 into the negatively charged pocket formed by E327-Oε, S200-Oγ, and one anionic oxygen of the dealkylated OP.
