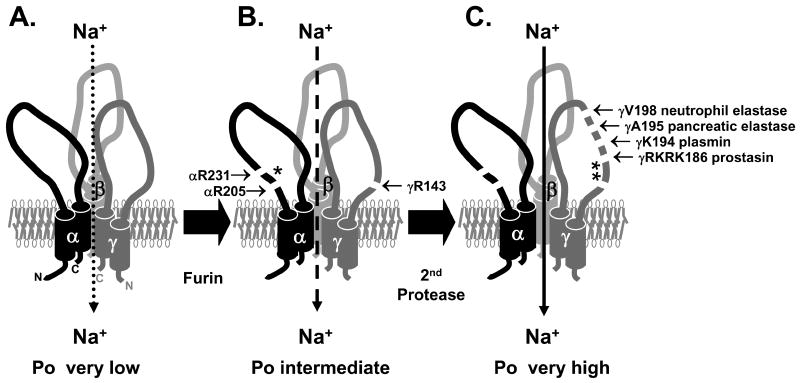
Figure 1. ENaC is activated by proteolytic cleavage and release of inhibitory peptides.
(A) Non-cleaved trimers of αβγENaC likely represent near-silent channels found on the cell surface with Po<0.1 (46-49). (B) αβγENaC is normally cleaved by furin as it transits the biosynthetic pathway and exhibits an intermediate Po (50). Furin cleaves α twice to release a 26-mer inhibitory peptide (*) and γ is cleaved once (51, 52). (C) Subsequent cleavage of the γ subunit at a site distal to the furin cleavage site, releases a second inhibitory peptide (**) and increases the Po to near 1 (46, 50, 53). Several proteases cleave γ at this distal site (20, 40, 50). For review, see (1, 5).