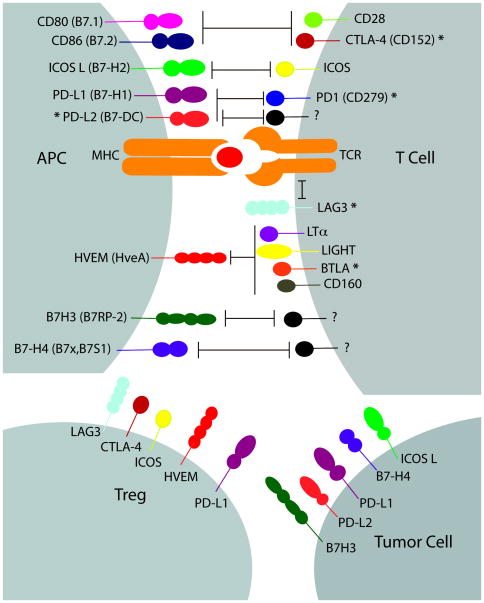
Figure 1.
T cell activation requires two signals; signal one is delivered through the T-cell receptor (TCR) by its engagement with a peptide–major histocompatibility complex (MHC), and signal two is delivered by engagement of either B7 family members or other co-stimulatory/co-inhibitory molecules, which are involved in fine-tuning T cell responses [31]. The binding of CD80 (B7.1) and CD86 (B7.2) to CD28 or CTLA-4 can lead to co-stimulation or co-inhibition signaling, respectively. Engagement of inducible costimulatory (ICOS) molecule and its ligand ICOSL can provide both negative and positive signals. The PD-1:PD-L1/PD-L2 pathway has inhibition effects on T cells. PD-L2 has also been shown to mediate co-stimulatory signals; however, its receptor has not been identified [31]. Lymphocyte-activation gene 3 (LAG-3) has been associated with inhibitory signaling through interactions with the TCR–CD3 complex [62]. HVEM can send positive or negative signals upon interaction with LIGHT and LTα or BTLA and CD160, respectively [63]. Recent studies have shown B7-H3 as a co-stimulator and co-inhibitor of T cell responses, while B7-H4 is a negative regulator of T cell responses. The receptors for B7-H3 and H7-H4 have not yet been identified [31]. Secondary signaling provided by B7 family members are also involved in the network of interactions between the tumor–stroma tissue and T cells. Human tumor cells have been reported to express co-inhibitory molecules, such as ICOSL, B7-H3, B7-H4, PD-L1 and PD-L2 [18,64,65]. Tregs also express co-inhibitory molecules, such as LAG-3, CTLA-4, ICOS, HVEM and PD-L1 [18]. *Blockage of these molecules has been shown to enhance in vivo anti-tumor immunity [36,42,62,64,65].