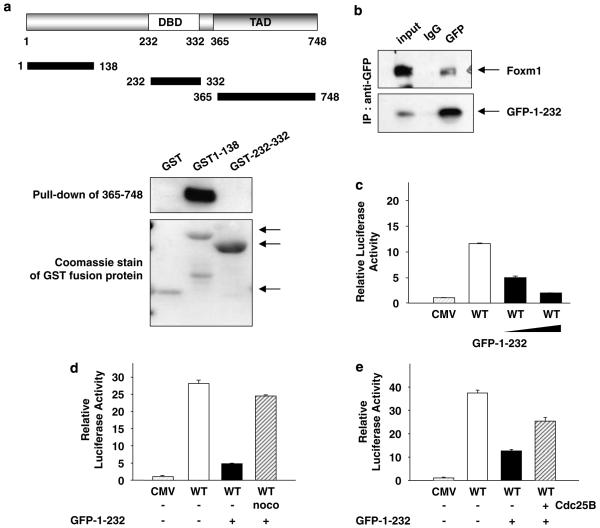
Figure 5.
The N-terminal domain of forkhead box m1 (FoxM1) interacts with its transactivation domain and inhibits its transcriptional activity. (a) Glutathione-S-transferase (GST)-fusion proteins containing various fragments of FoxM1 (GST1–138 or GST 232–335)were used in pull-down assay along with lysates from NIH3T3 cells transfected with a plasmid expressing T7-epitope-tagged transactivation domain of FoxM1 (residues between 365 and 748). The binding of T7-365–748 proteins was determined by western blot with T7 antibody. The lower panel shows a Coomassie staining of the GST-fusion proteins (arrows). (b) U2OS cells were transfected with plasmids expressing green fluorescent protein (GFP1–232) and T7-epitope-tagged FoxM1. The extracts were subjected to immunoprecipitation using GFP or a control antibody. The immunoprecipitates were analysed for T7-FoxM1 by western blot assays. (c and d) U2OS cells were transfected with 6XFoxM1 reporter plasmid and an increasing amount (c) or a constant amount (d) of the plasmid expressing the N-terminal fragment of FoxM1 (GFP1–232) in conjunction with a constant amount of T7-FoxM1. (d) Sixteen hours after transfection, cells were treated with nocodazole (noco) for another 16 h. (e) U2OS cells transfected with 6 × FoxM1-TATA luciferase reporter and plasmids expressing T7-FoxM1B, N-terminal fragment of FoxM1 (GFP1-232) and Cdc25B. Relative luciferase activity is shown.