Abstract
Purpose of the review
To summarize the recent advances in our understanding of the majors genes involved in chondrogenesis and their molecular mechanisms.
Recent findings
Disorders of the growth plate and the resulting skeletal dysplasias are a consequence of defects in genes involved in various stages of the chondrocyte proliferation and differentiation process. Recent identification of disease genes has provided insights into the pathophysiology of many skeletal dysplasias.
Summary
This knowledge enhances our understanding of the physiology and pathophysiology of the growth plate. Many skeletal dysplasias can now be characterized at the molecular level, allowing clinicians to provide accurate molecular diagnoses and counseling. Further research in this area will likely provide insights into possible therapeutic options for disorders of the growth plate.
Keywords: chondrocyte, growth plate, skeletal dysplasia, chondrocyte-specific transcription, extracellular matrix
Introduction
Linear growth results from endochondral bone elongation, which occurs mainly at the cartilaginous growth plate. Events at the growth plate required for bone growth occur in a predictable pattern that reflects the complex molecular events regulating chondrocyte proliferation, hypertrophy and accumulation of extracellular matrix (ECM). This complex process is a result of a balance between the expression of many genes encoding transcription factors, matrix proteins and mediators of cell-matrix interactions [1-3].
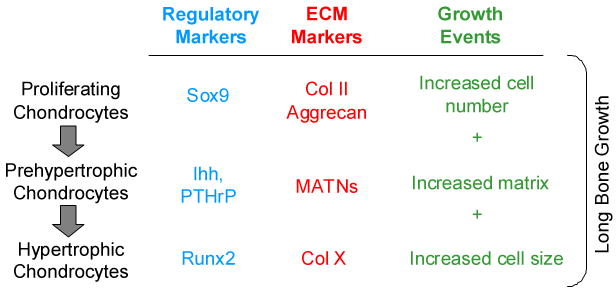
The processes of chondrocyte growth and differentiation occur in a spatially-defined relationship within the growth plate. As round, proliferative chondrocytes form a columnar layer; they stop proliferating and become prehypertrophic chondrocytes, which subsequently differentiate into post-mitotic, hypertrophic cells. These stages are defined by cellular phenotype and also by the ECM proteins the cells produce at each stage (Figure 1). Markers of proliferating chondrocytes are collagen II and Aggrecan. As cells progress through the developmental stages, expression of specific transcription factors and ECM proteins occurs in proliferating columnar cells, prehypertrophic and hypertrophic cells [4-6]. Collagen X is highly expressed in hypertrophic chondrocytes as they fully mature. As hypertrophic chondrocytes mature, osteoblast and vascular invasion take place and bone formation occurs. ECM deposited by proliferating and hypertrophic chondrocytes serves as a template for subsequent bone formation. Hypertrophic cells also secrete soluble proteins, such as vascular endothelial growth factor (VEGF) [7]. Hypertrophic chondrocytes ultimately undergo apoptosis, resulting in closure of the growth plate and cessation of long bone growth. Interactions between the three critical processes in the growth plate, chondrocyte proliferation, chondrocyte differentiation and hypertrophy, and ECM formation, are critical for normal long bone growth [8].
Figure 1.
The organization of the growth plate, showing the stages of chondrogenesis, the matrix constituents and the transcription factors that serve as markers for the stages of chondrocyte differentiation.
Defects in genes regulating these critical processes are associated with skeletal dysplasia and short stature. To date, there are over three hundred well defined disorders of skeletal growth and development [3;9]. Achondroplasia and osteogenesis imperfecta are among the most common skeletal dysplasias, while others are less common. Skeletal dysplasia has traditionally been a clinical and radiographic diagnosis.
Over the past decade, geneticists have utilized several strategies to identify the causative genes in many of the skeletal dysplasias.
Most skeletal dysplasias with known genetic etiology are monogenic heritable diseases with variable expressivity. The inheritance patterns are most often autosomal dominant and autosomal recessive. Genetic defects resulting in skeletal dysplasia can be divided into 2 major categories; defects in transcriptional control of skeletogesis and defects in genes involved in ECM formation. Transcriptional dysregulation often results in not only skeletal abnormalities, but in multiple organ system abnormalities [10]. At the cellular level, loss or gain of transcription factor function results in a pathological spectrum ranging from defects in cell proliferation, to dysregulated cell differentiation and cell survival [11]. The defects in ECM formation also result in wide range of skeletal dysplasias, perhaps owing to the fact that ECM proteins are a major component of the growth plate [12;13].
The most abundant ECM constituents in the growth plate are collagen types II, IX and XI. Type II collagens are covalently bound to type IX and XI fibrils. The non-collagenous ECM includes Matrilin (MATN) family members and cartilage oligomeric matrix protein (COMP).
Over the past several years, many of the molecular mechanisms of transcription factor action, chondrocyte proliferation and differentiation and regulation of ECM gene expression have been elucidated, thus explaining the pathophysiology underlying various skeletal dysplasias. This review will provide an update on genetic disorders regulating chondrocyte development within the growth plate.
Defects in the Early Stages of Chondrogenesis
Defects in regulatory markers
As mesenchymal cells migrate from the cranial neural crest and mesoderm and form skeletal elements, they start to express Col2a1, a marker for chondrocytes. The transcription factor Sox9 has an important role in specifying the commitment of mesenchymal cells to become chondrocytes [14]. Sox9 belongs to the SRY family, a characteristic of which is the presence of high mobility group box (HMG-box) DNA binding domains. Expression of Sox9 overlaps with that of Col2a1. In vitro and in vivo studies demonstrated the presence of chondrocyte-specific regulatory sequences in the first intron of the Col2a1 gene [15]. In addition to its ability to interact with this Col2a1 regulatory element, Sox9 binds as a homodimer to the consensus sequences of Col9a1, Col27a1 and MATN-1 [16-18]. Over expression of Sox9 in mice induces the expression of type II collagen [11;19]. The expression of Sox9 is regulated by fibroblast growth factor (FGF), bone morphogenic protein (BMP), members of the Wnt family, insulin-like growth factor-I (IGF-I) and transforming growth factor-β(TGF-β). Sox9 functions as a negative regulator of chondrocyte hypertrophy [11]. Heterozygous mutations in and around the Sox9 gene have been associated with campomelic dysplasia [20].
Defects in ECM
Collagen type II, along with collagens type IX and XI, is one of the major collagens expressed early in chondrogenesis. The collagen family is characterized by a coiled-coiled triple helix. Collagen type II is considered a fibril-forming collagen, a core element of the growth plate. Mutations in collagen type II results in either qualitative changes or quantitative changes depending on the location of the mutation. Therefore, clinical manifestations of collagen II defects vary from perinatal lethality to mild skeletal dysplasia. One consistent phenotype is an abnormality in the epiphysis. Disorders that are associated with mutations in collagen type II include spondyloepiphyseal dysplasia (SED), achondrogenesis type II or hypochondrogenesis, Kniest dysplasia and Stickler syndrome [21-24].
Defects in the Later Stages of Chondrogenesis
Defects in early regulatory markers in late stages of chondrogenesis
Once chondrocytes enter the prehypertrophic stage, they express Indian Hedgehog (Ihh). Ihh is a member of the hedgehog family of signaling molecules, which play a crucial role in regulating a variety of developmental processes. Ihh is one of the key signaling proteins controlling chondrocyte proliferation and hypertrophy in the developing skeletal system [25;26]. Ihh regulates the onset of hypertrophic differentiation by forming a negative feedback loop with PTHrP [27]. Ihh activates the expression of PTHrP, which then signals through its receptor to inhibit chondrocyte hypertrophy and suppress further Ihh expression by keeping chondrocytes in a proliferating state [28;29]. Ihh has also been found to promote chondrocyte proliferation and the transition from slowly proliferating periarticular chondrocytes to fast proliferating columnar chondrocytes independently of PTHrP signaling [6;28]. More recently, a PTHrP-independent role of Ihh signaling in regulating chondrocyte hypertrophy was demonstrated to be mediated through the activation of Wnt and BMP signaling [30]. Our own data indicate that Ihh is involved in the nutritional regulation of chondrogenesis in the growth plate [31]. Defects in the Ihh gene have been implicated in human skeletal dysplasias; brachydactyly type A1 [32] and acrocapital femoral dysplasia [33]. Recently, a study on genome-wide associations with adult height revealed that Ihh is one of the twenty genes for which variants are associated with adult height [34;35].
Ihh/PTHrP/Wnt/BMP signaling and interactions have been reviewed extensively by others [36-38]. Several defects within these pathways result in skeletal dysplasia. One of the downstream genes of Wnt is Wnt1-inducible signaling protein 3 (WISP3). Reduced WISP3 expression in a human chondrocyte cell line increased the amount of cellular reactive oxygen species (ROS) [39]. Over expression of WIPS3 inhibits BMP and Wnt signaling [40]. Mutations in WISP3 have been found in patients with progressive pseudorheumatoid chondrodysplasia (PPRC) [41]. In contrast, constitutively active mutations of the PTH/PTHrP receptor, which would presumably result in constitutive activation of the pathway's downstream targets, have been associated with metaphyseal chondrodysplasia, Jansen type.
Defects in ECM
COMP is a non-collagenous ECM protein highly expressed in cartilage. Collagen types I, II and IX bind to COMP with high affinity. Defects in the COMP gene have been identified in patients with pseudoachondroplasia and in patients with multiple epiphyseal dysplasia (MED) [42]. Overexpression of mutant COMP leads to decreased chondrocyte viability in vitro. The growth failure of patients with COMP defects is presumed to be due to increased cell death of growth-plate chondrocytes [43].
Collagen type IX is a heterotrimer mainly expressed in cartilage. Collagen type IX functions as a bridging collagen to other components in the cartilage matrix. MED type II is caused by mutations in the three genes encoding collagen type IX [44].
The MATN family of pericellular matrix proteins is comprised of four structurally related members, MATN-1, -2, -3 and -4). All contain one or two von Willebrand factor A (vWA) domains, at least one EGF-like motif and a C-terminal coiled-coiled domain, which allows the formation of homo and hetero-oligomers. All four MATNs are expressed in cartilage. Expression of MATN family members occurs early in chondrogenesis and persists throughout the process. The regulatory control of MATN family members is not well characterized, although the MATN-1 promoter has been shown to harbor Sox-9 binding sites [45]. The function of MATN proteins remains elusive. MATNs have been shown to bridge collagenous and non-collagenous matrix components. MATN-1, -3 and -4 are associated with collagen VI microfibrils [46] as well as other collagens [47;48]. Other non-collagenous molecules, such as COMP and decorin, also bind to MATNs with high affinity. Mutations in the human gene encoding MATN-3 are associated with MED. The effect of MATN-3 mutations is likely the result of an intracellular retention of the mutant protein in chondrocytes, leading eventually to chondrodysgenesis [49].
Defects in regulatory markers
Runx2 is a member of family of transcription factors that share the DNA-binding domain of the Drosophila “pair rule gene runt.” Runx2 is expressed in the late condensation stage of chondrogenesis. Its expression decreases in proliferating chondrocytes and increases again in hypertrophic chondrocytes [50]. Runx2 knock out mice lack hypertrophic chondrocytes in some but not all skeletal elements. Constitutive expression of Runx2 leads to ectopic chondrocyte hypertrophy in transgenic mice and allows chondrocyte hypertrophy to occur in Runx2-/- mice. Runx3 is also important for chondrocyte hypertrophy. Mice lacking both Runx2 and Runx3 do not have recognizable hypertrophic chondrocytes or collagen type X-expressing cells anywhere in the skeleton. Therefore, it seems clear that Runx2 and Runx3 are positive regulators of chondrocyte hypertrophy [51]. Heterozygous mutations in Runx2 are responsible for cleidocranial dysplasia (CCD).
Another transcription factor, Twist-1 is also important to chondrocyte hypertrophy. Its function requires Runx2. Twist-1 is a nuclear protein containing a basic helix-loop-helix domain and a so-called Twist-box at its C-terminus. Twist-1 is expressed in the cells that form the perichondrium that surround the growth plate and inhibit chondrocyte hypertrophy [52]. Defect in Twist-1 has been found in Saethre-Chotzen syndrome [53]. It has been demonstrated that Runx2 positively regulates fibroblast growth factor 18 (FGF18) in the perichondrium. FGF18 activates FGFR3 signaling and inhibits chondrocyte hypertrophy [52;54]. Activating mutations of FGFR3 cause achondroplasia, hypochondroplasia and thanatophoric dysplasia. The pathophysiological connection between Runx2, FGF18 and FGFR3 attests to the intricate interactions of transcription factor and growth factors within the growth plate [55;56].
As chondrocytes continue to differentiate, they become hypertrophic chondrocytes, which are characterized by the synthesis of collagen type X, which exists as a homotrimer under physiological circumstances. The triple helical domain is approximately half the size of that in collagen type II molecules. Mutations in the COL10A1 gene are responsible for Schmid metaphyseal chondrodysplasia. The mutant collagens present in this condition have been demonstrated to remain associated with the endoplasmic reticulum until degraded intracellularly by the proteasome and lysosome pathways [57;58].
C-natriuretic peptide (CNP) and its cognate receptor, the natriuretic peptide receptor B (NPRB), were originally implicated in the regulation of skeletal growth of transgenic and knockout mice [59]. More recently, mutations in the NPRB have been described in the autosomal recessive skeletal dysplasia known as acromesomelic dysplasia, type Maroteaux (AMDM). In chick chondrocytes, CNP increases the expression of collagen X and N-cadherin, the synthesis of glycosaminoglycan and chondrogenesis [60;61]. Expression of collagen II is unaffected, suggesting an important role of CNP in late chondrocyte differentiation. Pharmacologic inhibition of the p38 mitogen-activated protein (MAP) kinase blocks expansion of the hypertrophic zone of the growth plate, presumably by blocking the anabolic effects of CNP.
In humans, loss-of-function mutations of the short stature homoebox (SHOX) gene are associated with Leri-Weill dyschondrosteosis and Langer mesomelic dysplasia [62]. Most importantly, SHOX accounts for the skeletal dysplasia associated with Turner syndrome. SHOX mutations have also been identified as accounting for rare cases of “idiopathic short stature,” the phenotype of which tends to closely resemble that of Turner syndrome. At the molecular level, SHOX is upregulated in hypertrophic chondrocytes. In the murine model, deficiency of SHOX2, closely related to human SHOX, results in mesomelia. Histologically, SHOX2-/- mutants have a decreased number of hypertrophic chondrocytes, supporting the purported role of SHOX in chondrocyte differentiation [63;64].
Conclusion
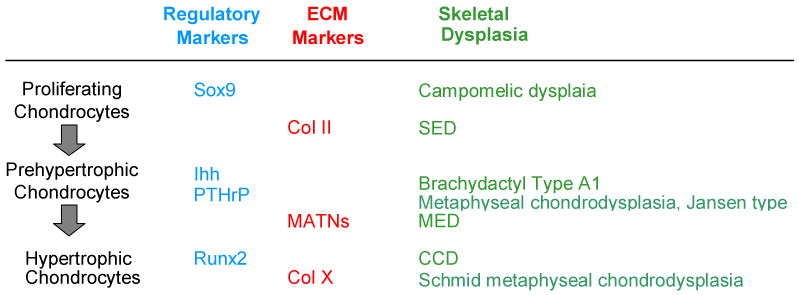
Our current knowledge of the molecular mechanisms of skeletal dysplasia has provided insights into the pathophysiology of specific skeletal defects as well as the normal process required for chondrocyte development, as broadly summarized in Figure 2. Defects in individual genes result in a spectrum of disease ranging from mild to severe and to various classes of skeletal dysplasia. It is intriguing that despite the critical role of each gene within the growth plate, mutations in a specific gene may not produce universal effects in every growth plate. This observation suggests that within each growth plate there is a threshold requirement for the particular gene. The precise pathophysiology accounting for this is yet to be characterized. As knowledge in this field advances, it is likely that we will identify more factors that regulate ciritical genes within growth plate. Defects in these genes will likely reduce the number of skeletal dysplasias with molecular defects that are presently unidentified.
Figure 2.
Examples of genetic defects in key chondrocyte regulators or ECMs during various stages of chondrogenesis resulting in specific skeletal dysplasia.
Reference List
- 1.Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- 2.Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- 3.Ballock RT, O'Keefe RJ. Physiology and pathophysiology of the growth plate. Birth Defects Res C Embryo Today. 2003;69:123–143. doi: 10.1002/bdrc.10014. [DOI] [PubMed] [Google Scholar]
- *4.Solomon LA, Berube NG, Beier F. Transcriptional regulators of chondrocyte hypertrophy. Birth Defects Res C Embryo Today. 2008;84:123–130. doi: 10.1002/bdrc.20124. [DOI] [PubMed] [Google Scholar]; Provide good overview related to chondrocyte hypertrophy
- 5.Nishimura R, Hata K, Ikeda F, Ichida F, Shimoyama A, Matsubara T, Wada M, Amano K, Yoneda T. Signal transduction and transcriptional regulation during mesenchymal cell differentiation. J Bone Miner Metab. 2008;26:203–212. doi: 10.1007/s00774-007-0824-2. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Kronenberg H. Minireview: transcriptional regulation in development of bone. Endocrinology. 2005;146:1012–1017. doi: 10.1210/en.2004-1343. [DOI] [PubMed] [Google Scholar]
- 7.Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- 8.Adams SL, Cohen AJ, Lassova L. Integration of signaling pathways regulating chondrocyte differentiation during endochondral bone formation. J Cell Physiol. 2007;213:635–641. doi: 10.1002/jcp.21262. [DOI] [PubMed] [Google Scholar]
- 9.Rimoin DL, Cohn D, Krakow D, Wilcox W, Lachman RS, Alanay Y. The skeletal dysplasias: clinical-molecular correlations. Ann N Y Acad Sci. 2007;1117:302–309. doi: 10.1196/annals.1402.072. [DOI] [PubMed] [Google Scholar]
- 10.Hermanns P, Lee B. Transcriptional dysregulation in skeletal malformation syndromes. Am J Med Genet. 2001;106:258–271. [PubMed] [Google Scholar]
- 11.Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18:213–219. doi: 10.1007/s10165-008-0048-x. [DOI] [PubMed] [Google Scholar]
- **12.Carter EM, Raggio CL. Genetic and orthopedic aspects of collagen disorders. Curr Opin Pediatr. 2009;21:46–54. doi: 10.1097/mop.0b013e32832185c5. [DOI] [PubMed] [Google Scholar]; Excellent review on common skeletal dysplasia
- *13.Posey KL, Hankenson K, Veerisetty AC, Bornstein P, Lawler J, Hecht JT. Skeletal abnormalities in mice lacking extracellular matrix proteins, thrombospondin-1, thrombospondin-3, thrombospondin-5, and type IX collagen. Am J Pathol. 2008;172:1664–1674. doi: 10.2353/ajpath.2008.071094. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provide new insight into pathophysiology of collagen IX defect.
- 14.Gordon CT, Tan TY, Benko S, Fitzpatrick D, Lyonnet S, Farlie PG. Long-range regulation at the SOX9 locus in development and disease. J Med Genet. 2009 doi: 10.1136/jmg.2009.068361. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Sakai D, Osumi N, Wada H, Wakamatsu Y. Sox genes regulate type 2 collagen expression in avian neural crest cells. Dev Growth Differ. 2006;48:477–486. doi: 10.1111/j.1440-169X.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 16.Rentsendorj O, Nagy A, Sinko I, Daraba A, Barta E, Kiss I. Highly conserved proximal promoter element harbouring paired Sox9-binding sites contributes to the tissue-and developmental stage-specific activity of the matrilin-1 gene. Biochem J. 2005;389:705–716. doi: 10.1042/BJ20050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genzer MA, Bridgewater LC. A Col9a1 enhancer element activated by two interdependent SOX9 dimers. Nucleic Acids Res. 2007;35:1178–1186. doi: 10.1093/nar/gkm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins E, Moss JB, Pace JM, Bridgewater LC. The new collagen gene COL27A1 contains SOX9-responsive enhancer elements. Matrix Biol. 2005;24:177–184. doi: 10.1016/j.matbio.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 20.Sock E, Pagon RA, Keymolen K, Lissens W, Wegner M, Scherer G. Loss of DNA-dependent dimerization of the transcription factor SOX9 as a cause for campomelic dysplasia. Hum Mol Genet. 2003;12:1439–1447. doi: 10.1093/hmg/ddg158. [DOI] [PubMed] [Google Scholar]
- 21.Ballo R, Beighton PH, Ramesar RS. Stickler-like syndrome due to a dominant negative mutation in the COL2A1 gene. Am J Med Genet. 1998;80:6–11. doi: 10.1002/(sici)1096-8628(19981102)80:1<6::aid-ajmg2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Bogaert R, Tiller GE, Weis MA, Gruber HE, Rimoin DL, Cohn DH, Eyre DR. An amino acid substitution (Gly853-->Glu) in the collagen alpha 1(II) chain produces hypochondrogenesis. J Biol Chem. 1992;267:22522–22526. [PubMed] [Google Scholar]
- 23.Korkko J, Cohn DH, Ala-Kokko L, Krakow D, Prockop DJ. Widely distributed mutations in the COL2A1 gene produce achondrogenesis type II/hypochondrogenesis. Am J Med Genet. 2000;92:95–100. doi: 10.1002/(sici)1096-8628(20000515)92:2<95::aid-ajmg3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura G, Haga N, Kitoh H, Tanaka Y, Sonoda T, Kitamura M, Shirahama S, Itoh T, Nakashima E, Ohashi H, Ikegawa S. The phenotypic spectrum of COL2A1 mutations. Hum Mutat. 2005;26:36–43. doi: 10.1002/humu.20179. [DOI] [PubMed] [Google Scholar]
- 25.Lai LP, Mitchell J. Indian hedgehog: its roles and regulation in endochondral bone development. J Cell Biochem. 2005;96:1163–1173. doi: 10.1002/jcb.20635. [DOI] [PubMed] [Google Scholar]
- 26.Kronenberg HM, Chung U. The parathyroid hormone-related protein and Indian hedgehog feedback loop in the growth plate. Novartis Found Symp. 2001;232:144–152. doi: 10.1002/0470846658.ch10. [DOI] [PubMed] [Google Scholar]
- 27.Kronenberg HM. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest. 2005;115:1734–1742. doi: 10.1172/JCI24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broadus AE, Macica C, Chen X. The PTHrP functional domain is at the gates of endochondral bones. Ann N Y Acad Sci. 2007;1116:65–81. doi: 10.1196/annals.1402.061. [DOI] [PubMed] [Google Scholar]
- *30.Mak KK, Kronenberg HM, Chuang PT, Mackem S, Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135:1947–1956. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article provides insight into the complicated interaction of important regulators in the growth plate.
- 31.Kim MS, Wu KY, Auyeung V, Chen Q, Gruppuso PA, Phornphutkul C. Leucine restriction inhibits chondrocyte proliferation and differentiation through mechanisms both dependent and independent of mTOR signaling. Am J Physiol Endocrinol Metab. 2009;296:E1374–E1382. doi: 10.1152/ajpendo.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao B, Guo J, She C, Shu A, Yang M, Tan Z, Yang X, Guo S, Feng G, He L. Mutations in IHH, encoding Indian hedgehog, cause brachydactyly type A-1. Nat Genet. 2001;28:386–388. doi: 10.1038/ng577. [DOI] [PubMed] [Google Scholar]
- 33.Hellemans J, Coucke PJ, Giedion A, De Paepe A, Kramer P, Beemer F, Mortier GR. Homozygous mutations in IHH cause acrocapitofemoral dysplasia, an autosomal recessive disorder with cone-shaped epiphyses in hands and hips. Am J Hum Genet. 2003;72:1040–1046. doi: 10.1086/374318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **34.Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, Sanna S, Eyheramendy S, Voight BF, Butler JL, Guiducci C, Illig T, Hackett R, Heid IM, Jacobs KB, Lyssenko V, Uda M, Boehnke M, Chanock SJ, Groop LC, Hu FB, Isomaa B, Kraft P, Peltonen L, Salomaa V, Schlessinger D, Hunter DJ, Hayes RB, Abecasis GR, Wichmann HE, Mohlke KL, Hirschhorn JN. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet. 2008;40:584–591. doi: 10.1038/ng.125. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides the association between Ihh and human stature, supporting the important function of Ihh.
- **35.Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, Mangino M, Freathy RM, Perry JR, Stevens S, Hall AS, Samani NJ, Shields B, Prokopenko I, Farrall M, Dominiczak A, Johnson T, Bergmann S, Beckmann JS, Vollenweider P, Waterworth DM, Mooser V, Palmer CN, Morris AD, Ouwehand WH, Zhao JH, Li S, Loos RJ, Barroso I, Deloukas P, Sandhu MS, Wheeler E, Soranzo N, Inouye M, Wareham NJ, Caulfield M, Munroe PB, Hattersley AT, McCarthy MI, Frayling TM. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40:575–583. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides the association between Ihh and human stature, supporting the important function of Ihh.
- *36.Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90 1:19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]; This article provides good summary of current interconnection of Wnt and Ihh.
- 37.Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. J Cell Biochem. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- 38.Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3:439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 39.Davis L, Chen Y, Sen M. WISP-3 functions as a ligand and promotes superoxide dismutase activity. Biochem Biophys Res Commun. 2006;342:259–265. doi: 10.1016/j.bbrc.2006.01.132. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y, Weidinger G, Liang JO, Aquilina-Beck A, Tamai K, Moon RT, Warman ML. The CCN family member Wisp3, mutant in progressive pseudorheumatoid dysplasia, modulates BMP and Wnt signaling. J Clin Invest. 2007;117:3075–3086. doi: 10.1172/JCI32001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kutz WE, Gong Y, Warman ML. WISP3, the gene responsible for the human skeletal disease progressive pseudorheumatoid dysplasia, is not essential for skeletal function in mice. Mol Cell Biol. 2005;25:414–421. doi: 10.1128/MCB.25.1.414-421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Posey KL, Hecht JT. The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr Drug Targets. 2008;9:869–877. doi: 10.2174/138945008785909293. [DOI] [PubMed] [Google Scholar]
- *43.Alcorn JL, Merritt TM, Farach-Carson MC, Wang HH, Hecht JT. Ribozyme-mediated reduction of wild-type and mutant cartilage oligomeric matrix protein (COMP) mRNA and protein. RNA. 2009;15:686–695. doi: 10.1261/rna.1335909. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provide a possible molecular mechanism of defect in COMP.
- 44.Blumbach K, Niehoff A, Paulsson M, Zaucke F. Ablation of collagen IX and COMP disrupts epiphyseal cartilage architecture. Matrix Biol. 2008;27:306–318. doi: 10.1016/j.matbio.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Karcagi I, Rauch T, Hiripi L, Rentsendorj O, Nagy A, Bosze Z, Kiss I. Functional analysis of the regulatory regions of the matrilin-1 gene in transgenic mice reveals modular arrangement of tissue-specific control elements. Matrix Biol. 2004;22:605–618. doi: 10.1016/j.matbio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Wagener R, Ehlen HW, Ko YP, Kobbe B, Mann HH, Sengle G, Paulsson M. The matrilins--adaptor proteins in the extracellular matrix. FEBS Lett. 2005;579:3323–3329. doi: 10.1016/j.febslet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Mann HH, Ozbek S, Engel J, Paulsson M, Wagener R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J Biol Chem. 2004;279:25294–25298. doi: 10.1074/jbc.M403778200. [DOI] [PubMed] [Google Scholar]
- 48.Mann HH, Sengle G, Gebauer JM, Eble JA, Paulsson M, Wagener R. Matrilins mediate weak cell attachment without promoting focal adhesion formation. Matrix Biol. 2007;26:167–174. doi: 10.1016/j.matbio.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Chapman KL, Mortier GR, Chapman K, Loughlin J, Grant ME, Briggs MD. Mutations in the region encoding the von Willebrand factor A domain of matrilin-3 are associated with multiple epiphyseal dysplasia. Nat Genet. 2001;28:393–396. doi: 10.1038/ng573. [DOI] [PubMed] [Google Scholar]
- 50.Komori T. Regulation of bone development and maintenance by Runx2. Front Biosci. 2008;13:898–903. doi: 10.2741/2730. [DOI] [PubMed] [Google Scholar]
- *51.Lou Y, Javed A, Hussain S, Colby J, Frederick D, Pratap J, Xie R, Gaur T, van Wijnen AJ, Jones SN, Stein GS, Lian JB, Stein JL. A Runx2 threshold for the cleidocranial dysplasia phenotype. Hum Mol Genet. 2009;18:556–568. doi: 10.1093/hmg/ddn383. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provide insight into molecular mechanism of Runx2 defect.
- 52.Hinoi E, Bialek P, Chen YT, Rached MT, Groner Y, Behringer RR, Ornitz DM, Karsenty G. Runx2 inhibits chondrocyte proliferation and hypertrophy through its expression in the perichondrium. Genes Dev. 2006;20:2937–2942. doi: 10.1101/gad.1482906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kress W, Schropp C, Lieb G, Petersen B, Busse-Ratzka M, Kunz J, Reinhart E, Schafer WD, Sold J, Hoppe F, Pahnke J, Trusen A, Sorensen N, Krauss J, Collmann H. Saethre-Chotzen syndrome caused by TWIST 1 gene mutations: functional differentiation from Muenke coronal synostosis syndrome. Eur J Hum Genet. 2006;14:39–48. doi: 10.1038/sj.ejhg.5201507. [DOI] [PubMed] [Google Scholar]
- 54.Reinhold MI, Naski MC. Direct interactions of Runx2 and canonical Wnt signaling induce FGF18. J Biol Chem. 2007;282:3653–3663. doi: 10.1074/jbc.M608995200. [DOI] [PubMed] [Google Scholar]
- 55.Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370:162–172. doi: 10.1016/S0140-6736(07)61090-3. [DOI] [PubMed] [Google Scholar]
- 56.L'Hote CG, Knowles MA. Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Exp Cell Res. 2005;304:417–431. doi: 10.1016/j.yexcr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Bateman JF, Wilson R, Freddi S, Lamande SR, Savarirayan R. Mutations of COL10A1 in Schmid metaphyseal chondrodysplasia. Hum Mutat. 2005;25:525–534. doi: 10.1002/humu.20183. [DOI] [PubMed] [Google Scholar]
- 58.Bateman JF, Freddi S, Nattrass G, Savarirayan R. Tissue-specific RNA surveillance? Nonsense-mediated mRNA decay causes collagen X haploinsufficiency in Schmid metaphyseal chondrodysplasia cartilage. Hum Mol Genet. 2003;12:217–225. doi: 10.1093/hmg/ddg054. [DOI] [PubMed] [Google Scholar]
- *59.Bocciardi R, Ravazzolo R. C-type natriuretic peptide and overgrowth. Endocr Dev. 2009;14:61–66. doi: 10.1159/000207477. [DOI] [PubMed] [Google Scholar]; This article provides an update role of CNP in bone growth.
- 60.Pejchalova K, Krejci P, Wilcox WR. C-natriuretic peptide: an important regulator of cartilage. Mol Genet Metab. 2007;92:210–215. doi: 10.1016/j.ymgme.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Woods A, Khan S, Beier F. C-type natriuretic peptide regulates cellular condensation and glycosaminoglycan synthesis during chondrogenesis. Endocrinology. 2007;148:5030–5041. doi: 10.1210/en.2007-0695. [DOI] [PubMed] [Google Scholar]
- 62.Blaschke RJ, Rappold G. The pseudoautosomal regions, SHOX and disease. Curr Opin Genet Dev. 2006;16:233–239. doi: 10.1016/j.gde.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Yu L, Liu H, Yan M, Yang J, Long F, Muneoka K, Chen Y. Shox2 is required for chondrocyte proliferation and maturation in proximal limb skeleton. Dev Biol. 2007;306:549–559. doi: 10.1016/j.ydbio.2007.03.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cobb J, Dierich A, Huss-Garcia Y, Duboule D. A mouse model for human short-stature syndromes identifies Shox2 as an upstream regulator of Runx2 during long-bone development. Proc Natl Acad Sci USA. 2006;103:4511–4515. doi: 10.1073/pnas.0510544103. [DOI] [PMC free article] [PubMed] [Google Scholar]