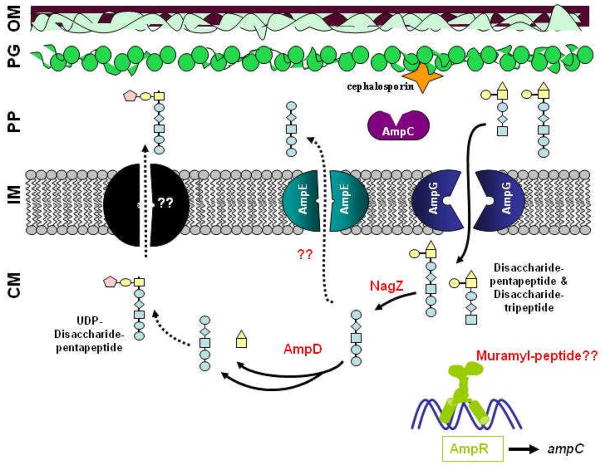
Fig. 6.
Mechanism of β-lactamase induction in Enterobacteriaceae. In the absence of β-lactam antibiotics, the murein is constantly being re-modeled for cell elongation and cell division. The breakdown products, muramyl peptides, are released into the periplasm and transported to the cytoplasm via AmpG. These muramyl peptides are then processed by ampD for the murein recycling. In this instance, the ampC gene is kept at basal-level by a suppressed AmpR transcriptional regulator. In the presence of β-lactam antibiotics, the homeostasis is disrupted because these antibiotics target the murein resulting in an excessive breakdown of the murein and an accumulation of the muramyl peptides both in the periplasm and cytoplasm. However, the AmpD protein is the limiting factor. An accumulation of the muramyl peptides renders the activation of AmpR, which then induces the production of AmpC β-lactamase. It is not clear which of the muramyl products directly interact with AmpR. AmpE has been implicated in the transport of free pentapeptide to the periplasm (40). The transport of the muramyl peptides into the periplasm may involve AmpE or some yet to be unidentified protein. Abbreviations, CM, IM, PP, PG and OM refer to cytoplasm, inner membrane, periplasm, peptidoglycan and outer membrane, respectively.