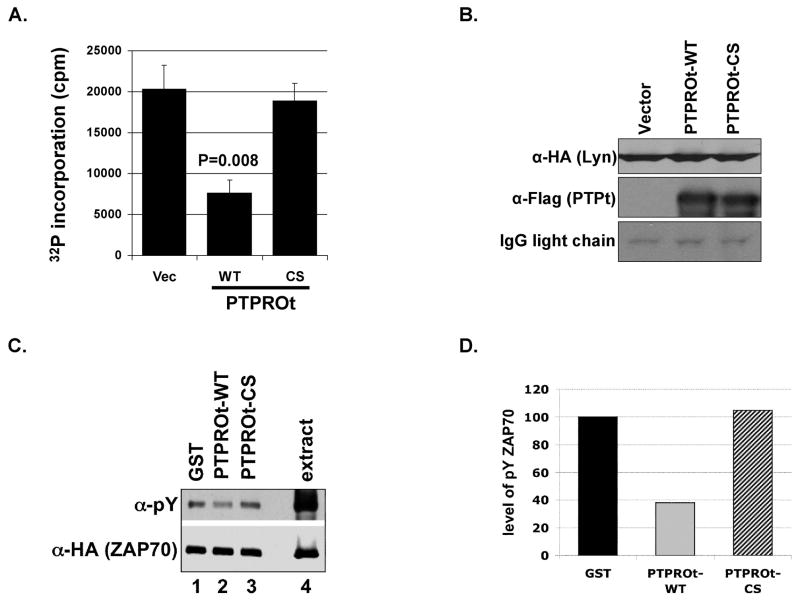
Fig. 4. Inactivation of Lyn and dephosphorylation of ZAP70 by PTPROt.
(A) Activity of Lyn kinase immunoprecipitated from whole cell extracts of H293T cells co-expressing HA-tagged Lyn and Flag-tagged PTPROt was measured using a peptide substrate. The activity is represented as a measure of 32P incorporation into the peptide following incubation of immunoprecipitated Lyn with peptide and 32P-gammaATP (in cpm). The experiment was repeated twice with triplicate measurements. (B) The co-immunoprecipitated Lyn-HA and PTPROt (WT, CS) used for kinase assay were separated on SDS-PAGE followed by Western blot analysis with anti-HA (for Lyn, top panel) and anti-Flag (for PTPROt, middle panel). (C) Whole cell extract from pervanadate treated H293T cells over-expressing HA-tagged ZAP70 was incubated with either GST alone or GST-tagged PTPROt (WT or CS) under conditions optimal for phosphatase reaction. Following the reaction, ZAP70 was immunoprecipated with anti-HA antibody. The immunoprecipitated proteins were separated on SDS-PAGE and immunoblotted with anti-pY cocktail and anti-HA antibody. (D) Quantification of ZAP70 phosphorylation normalized to total ZAP70 upon incubation with GST alone or GST-PTPROt (WT or CS). Scanned images were quantified using KODAK imaging software. The experiment was repeated three times with similar observation. One representative data was quantified for illustration.