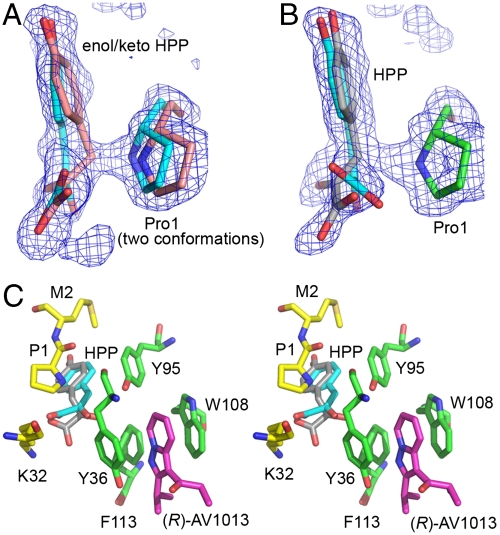
Fig. 5.
rhMIF-HPP-(R)AV1013 ternary complex. (A) An active site with only HPP bound. HPP can be modeled as the enol (carbon atoms in cyan) and keto (carbon atoms in pink) form within the same active site, suggesting that interconversion between these two forms occurs in the active site. The simulated annealing electron density displayed in A and B is 3.0σ Fo-Fc density calculated omitting all of substrate molecules as well as Pro1. (B) The ternary complex with HPP in the active site and (R)-AV1013 in the neighboring allosteric site. (C) Proximity of allosterically bound (R)-AV1013 to the active site bound HPP shown in stereo with carbon atoms of the protein shown in green for the allosteric site and yellow for the active site, those of the inhibitor shown in magenta, those of the enol form of HPP in cyan, and those of the Pro1 proximal HPP in gray. The conformational shift of Tyr36 observed in the binary structure (Fig. 4B) is also seen here in the ternary complex in the (R)-AV1013-bound subunit.