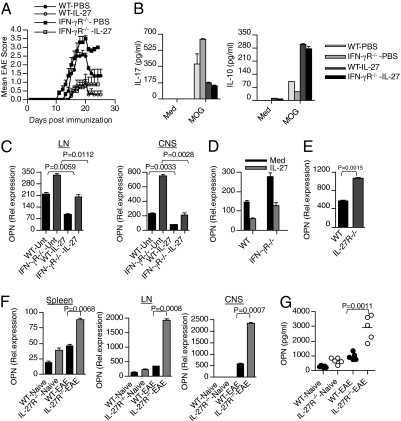
Fig. 6.
IL-27 treatment inhibits clinical severity of EAE in IFN-γR−/− mice. (A) IL-27 inhibits clinical severity of EAE in WT and IFN-γR−/− mice. WT and IFN-γR−/− mice (n = 6) were immunized with MOG peptide emulsified in complete freund's adjuvant. Recombinant IL-27 (0.25 μg per mouse) was administered by s.c. to immunized WT and IFN-γR−/− mice every other day from day 2 until day 18. (B) LN cells from IL-27-treated and control mice were activated in vitro with MOG35–55 (20 μg/mL) for 72 h, and cell-free culture supernatants were assayed for IL-17 and IL-10 by ELISA. (C) Real-time RT-PCR analysis of OPN in DCs isolated from LN and CNS of WT and IFN-γR−/− mice (n = 5–6 per group) treated with or without IL-27. (D) Real-time RT-PCR analysis of OPN in DCs isolated from spleen of naïve WT and IFN-γR−/− mice treated with or without rIL-27 (100 ng/mL). (E) Real-time RT-PCR analysis of OPN in DCs isolated from spleen of naïve WT and IL-27R−/− mice. (F) Real-time RT-PCR analysis of OPN in DCs isolated from spleen, LN, and CNS of naïve and EAE bearing WT and IL-27R−/− mice (n = 6 per group). (G) ELISA of serum OPN from WT and IL-27R−/− mice with or without EAE.