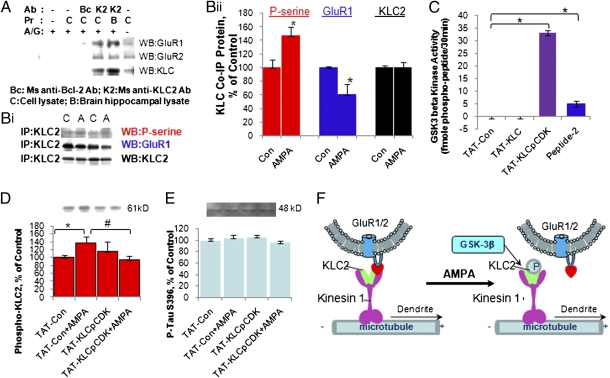
Fig. 2.
KLC2 phosphorylation by GSK-3 led to dissociation of GluR1 from KLC2. (A) Coimmunoprecipitation of GluR1 and GluR2 with KLC. (B, i and ii) AMPA (A) enhanced serine phosphorylation of KLC2 and reduced KLC2/GluR1 complex compared with control (C) (Student's t test, unpaired, two-tailed, N = 2–4, n = 5–9; for p-serine, P = 0.0287; for GluR1, P = 0.040; for KLC2, P = 0.8514). (C) GSK-3β phosphorylated peptide TAT-KCLpCDK (one-way ANOVA, N = 2, n = 24, Tukey's multiple comparison test, **P < 0.001). (D) TAT-KLCpCDK significantly inhibited serine phosphorylation of KLC2 induced by AMPA (Student's t test, unpaired, two-tailed, N = 2–3, n = 5–9; Tat-Con versus Tat-Con + AMPA, *P = 0.045; TAT-Con + AMPA versus TAT-KLCpCDK + AMPA, #P = 0.024). (E) TAT-KLCpCDK had no effect on phosphorylation of Tau S396. (F) Regulation of GluR1/2 receptor trafficking by GSK-3 via KLC2 phosphorylation.