Figure 4.
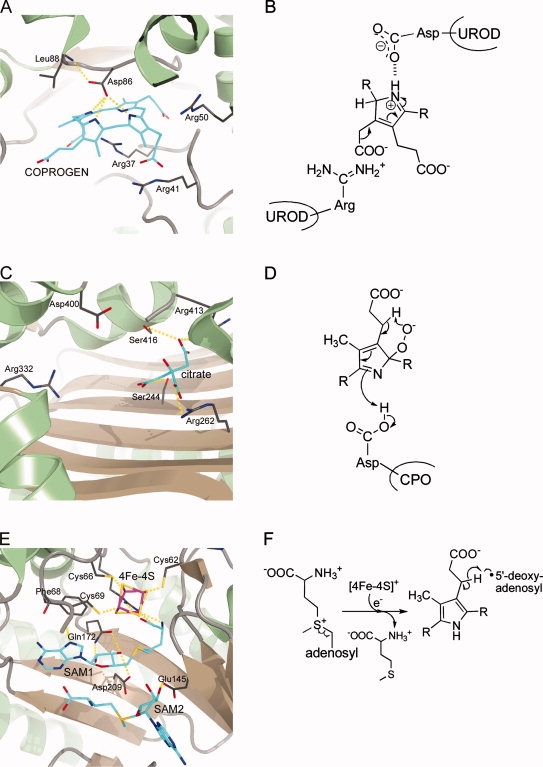
Active site architectures and catalytic steps of enzymes involved in protoporphyrinogen IX formation. A + B, UROD; C + D, CPO; E + F, CPDH. A: Active site of human UROD with bound coproporphyrinogen III (COPROGEN) in a dome-shaped conformation showing the catalytically essential aspartate in hydrogen bonding distance to the pyrrole NH-groups. B: The aspartate residue stabilizes the protonated, positively charged reaction intermediate. C: Active site of human CPO with bound citrate showing several arginine residues possibly involved in substrate binding and the catalytically essential aspartate. D: During catalysis, a pyrrole peroxide anion is formed which further reacts via proton abstraction through the peroxide to form an intermediate containing an exocyclic double bond. E: Active site of E. coli CPDH showing the catalytically essential [4Fe-4S] cluster and the two bound SAM molecules. F: During the initial reaction steps the reduced iron-sulfur cluster transfers an electron to SAM, which is thereby cleaved into methionine and a 5′-deoxyadenosyl radical. This radical then abstracts a hydrogen atom from the substrate propionate side chain resulting in the formation of 5′-deoxyadenosine and a substrate radical. R = tetrapyrrole.
