Figure 3.
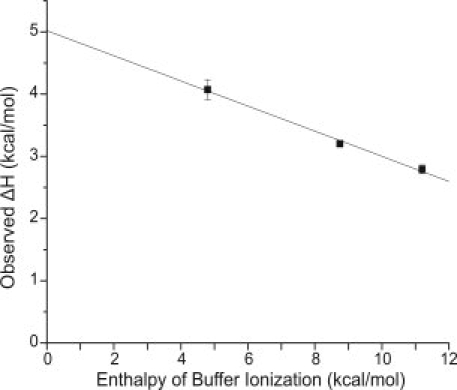
Dependence of observed ΔH on heat of ionization of buffers. The buffers used and their corresponding enthalpy of ionization at 8°C were Hepes, 4.8 kcal/mol; imidazole, 8.75 kcal/mol; and Tris, 11.5 kcal/mol. The solid line is the linear regression analysis that gives a slope of −0.2 (the number of protons released to solvent per mole of complex formed) and an intercept of ∼5 kcal/mol (the corrected ΔH of binding). The titration in Tris was carried out four times, in imidazole once, and in Hepes twice. The standard deviation is shown. The standard deviation is contained within the data point for Tris.
