Figure 1.
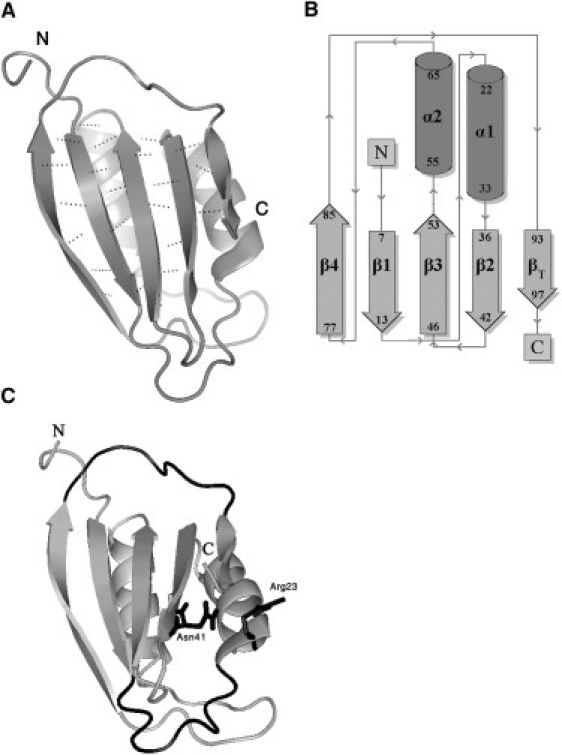
Structure of AcP. (A) Solution structure of horse muscle AcP (PDB code: 1APS (21)). Secondary structures (defined according to DSSP) are represented as ribbons, and backbone hydrogen bonds in the β-sheet are shown as dashed lines. The force-bearing units are the N- and C-terminal strands. (B) Topology diagram. AcP adopts a rather uncommon α/β sandwich fold elaborated by two intercalating βαβ units forming an antiparallel β-sheet with a 4-1-3-2-5(βT) strand topology. (C) Structural determinants for forced unfolding of AcP. The long loop that follows the N-terminal, force-bearing strand, and the loop that precedes the C-terminal one (βT) are shown in black (bottom and top, respectively). The former, referred to as the catalytic loop, adopts a cradle-like conformation and constitutes the active site of the enzyme. Also shown are the conserved Arg-23 and Asn-41 residues, which flank the cradle and function in binding the substrate phosphate group and the catalytic water molecule, respectively.
