Abstract
Objective
The most commonly used classification system for endometrial hyperplasia is the World Health Organization system which is based on subjective criteria. Another classification system is endometrial intraepithelial neoplasia (EIN) system which uses diagnostic criteria including cytological demarcation, crowded gland architecture, minimum size of 1 mm, and careful exclusion of mimics, and aims to identify a precancer or cancer. The objective of this study was to compare the two classification systems in terms of predicting the presence of a coexistent cancer in surgically treated patients.
Methods
Biopsy and hysterectomy specimens of 49 women who were subjected to surgery with a preoperative diagnosis of endometrial hyperplasia (EH) according to the WHO system were re-evaluated retrospectively by using EIN system.
Results
Among the 49 patients, 69.4% had complex atypical EH and 75.5% had EIN at biopsy specimens. EIN was detected in 94.1% of complex atypical EH, and 41.7% of non-atypical EH. Nine women (18.4%) had endometrial cancer. Among women with cancer, all had complex atypical EH or EIN. The rate of coexistent endometrial cancer was 26.5% in women with complex atypical EH and 24.3% in women with EIN.
Conclusion
Diagnoses of atypical or complex atypical EH and EIN had similar sensitivities and negative predictive values in predicting the coexistent endometrial cancer. Either of these two classification systems may be used safely when an experienced pathologist is available. However, use of the objective EIN system may be preferred whenever possible to prevent diagnostic errors in centers where an experienced pathologist is not available.
Keywords: Endometrial hyperplasia, Endometrial intraepithelial neoplasia, Endometrial cancer, Coexistent cancer
INTRODUCTION
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries.1 Endometrioid type adenocarcinoma accounts for 75 to 80% of cases, and is associated with long-term unopposed estrogenic stimulation of the endometrium.2 This estrogenic stimulation results in endometrial hyperplasia (EH) which is the precursor lesion of most ECs of endometrioid type.3
EH is characterized by non-physiological proliferation of endometrium that results in glands with irregular shapes and varying sizes.4 The most commonly used classification system for EH is the World Health Organization (WHO) 1994 classification system, in which architectural disruption and cytological atypia are used to identify four types of EH, including simple or complex hyperplasia with or without atypia.5 Especially, cytological atypia is of great consideration, not only for the progression to EC, but also for the risk of a coexistent EC in women with EH.6,7 Therefore, the correct identification of EH type is important since the presence or absence of atypia guides the clinical management.8
On the other hand, there is considerable interobserver and intraobserver variation in the diagnosis and typing of EH, because the diagnostic criteria of the WHO classification are largely subjective.9-11 For this reason, an endometrial intraepithelial neoplasia (EIN) classification based on molecular genetics and computerized morphometric analysis was introduced to identify patients at risk of having real precancer or cancer, and to facilitate proper and more uniform patient management.12 During routine practice, the diagnosis of EIN is achieved by using hematoxylin-eosin stained sections. The diagnostic criteria include the presence of cytological demarcation, crowded gland architecture, minimum size of 1 mm, and careful exclusion of mimics.13
The aim of this study is to compare two classification systems of EH in terms of predicting the presence of a coexistent endometrial cancer in patients treated with hysterectomy.
MATERIALS AND METHODS
A review of Hacettepe University Faculty of Medicine gynecology and pathology database was performed in order to identify the patients who were subjected to hysterectomy within 2 weeks following a diagnosis of EH between January 2007 and January 2009. The study was exempt from Institutional Review Board approval since it was a retrospective design. The clinical and pathological characteristics of these patients were obtained using medical records. All patients underwent endometrial sampling via Karman aspiration (used in premenopausal women) or curettage (used in postmenopausal women) at Hacettepe University Hospital due to abnormal uterine bleeding. The preoperative EH diagnoses were achieved by using the 1994 WHO classification based on the presence or absence of cytological atypia, and simple or complex architecture.5 The co-author pathologist who was blinded to the initial pathologic results re-evaluated the biopsy and hysterectomy specimens, retrospectively, according to the EIN classification. Owing to the fact that the knowledge for status of the hysterectomy specimen may affect the re-evaluation result of the biopsy specimen, the hysterectomy specimens were re-evaluated after finishing the re-evaluation of all biopsy specimens. As described by Hecht et al.,13 the areas diagnosed as EIN were required to meet the following criteria: architecture (area of glands exceeds area of stroma), cytological alterations (epithelial cells within the architecturally crowded focus are cytologically different compared to background), lesional size (the maximal linear dimension of the lesion should exceed 1 mm), and exclusion of benign mimics and carcinoma. The two classifications were compared in terms of predicting the presence of a coexistent EC in women with EH. Chi-square and Fisher's exact tests were used, as appropriate, to compare nominal variables. All analyses were performed using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA), and p-values <0.05 were considered significant.
RESULTS
Forty nine patients were eligible with a mean age of 51.5 years (range, 36 to 79 years). Nineteen (38.8%) presented with postmenopausal bleeding, while the remaining 30 (61.2%) were premenopausal and presented with menorrhage and/or metrorrhage.
Biopsy results included complex EH without atypia in 24.5%, simple EH with atypia in 6.1%, and complex EH with atypia in 69.4%. Overall, according to WHO classification, 93.9% of patients had complex EH and 75.5% had atypical EH.
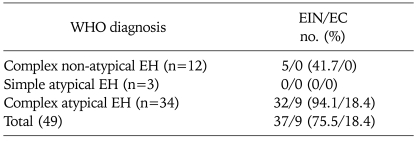
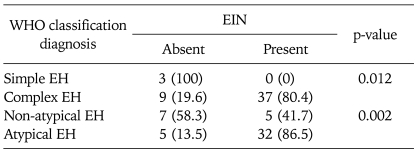
According to the EIN classification, 37 of 49 patients (75.5%) had endometrial intraepithelial neoplasia in the preoperative biopsy specimens. Incidence of EIN was 41.7%, 0%, and 94.1% in patients with complex EH without atypia, simple EH with atypia, and complex EH with atypia, respectively (Table 1). EIN was detected in 86.5% of EH with atypia, and 41.7% of EH without atypia (p=0.002). EIN was seen in 0% of cases with simple EH, and 80.4% in cases with complex EH (p=0.012) (Table 2).
Table 1.
Presence of EIN and EC in different categories of WHO classification
WHO: World Health Organization, EH: endometrial hyperplasia, EIN: endometrial intraepithelial neoplasia, EC: endometrial cancer.
Table 2.
Presence of EIN in simple vs. complex EH and non-atypical vs. atypical EH
Values are presented as number (%).
WHO: World Health Organization, EIN: endometrial intraepithelial neoplasia, EH: endometrial hyperplasia.
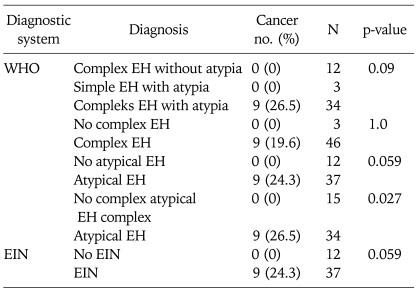
Nine patients (18.4%) had EC at hysterectomy in this cohort. The initial diagnosis of EC was confirmed in all women after re-evaluation. Among 34 cases with complex atypical EH, 9 (26.5%) had EC at hysterectomy, while the rate of EC was 0% among 15 cases without complex atypical EH (p=0.027). EC was detected in 9 of 37 cases (24.3%) with EIN, and in none of 13 cases without EIN (p=0.059) (Table 3).
Table 3.
Endometrial cancers according to biopsy results
WHO: World Health Organization, EIN: endometrial intraepithelial neoplasia, EH: endometrial hyperplasia.
Among the total, 49.0% of hysterectomy specimens contained EIN. While only 37.5% of patients without EC had EIN, all patients with EC had EIN in the hysterectomy specimens (p=0.001).
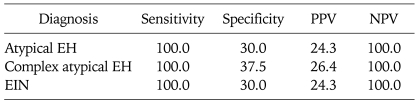
For the prediction of coexistent EC, the sensitivity and specificity of atypical EH was 100% and 30.0%, respectively. The sensitivity and specificity of complex atypical EH was 100% and 37.5%, respectively. When EIN was detected at biopsy, its sensitivity for predicting coexistent EC was 100% and its specificity was 30.0%. The negative predictive values were 100% in all three diagnoses (Table 4).
Table 4.
Performance of different diagnoses for the prediction of coexistent EC
Values are presented as persentage.
EC: endometrial cancer, EH: endometrial hyperplasia, EIN: endometrial intraepithelial neoplasia, PPV: positive predictive value, NPV: negative predictive value.
DISCUSSION
EH which is the precursor lesion of most endometrial cancers of endometrioid type is usually diagnosed through evaluation of women with abnormal uterine bleeding by endometrial biopsy.3,14 Despite several advances in non-invasive techniques to detect coexistent EC or risk of progression to EC during the initial diagnosis of EH, currently available studies failed to reveal conclusive results.15 According to the widely used WHO classification, patients with atypical EH are at risk of developing EC when left untreated. Although some of these lesions may coexist with EC at the time of EH diagnosis, others may progress to EC in course of time. Also, among patients with atypical EH, the risk of coexisting EC or to progress to EC is greater when the architecture is complex.6 Therefore, the majority of women with complex atypical EH who do not have desire for further fertility are treated by hysterectomy. On the other hand, patients with non-atypical EH undergo treatment including surgical and non-surgical management strategies that indicates the lack of consensus for the management of such patients.8 Also, there may be some difficulties of differential diagnosis between atypical EH and well-differentiated EC.16 Thus, it appears that some problems arise during diagnostic processes, which consequently influence clinical management significantly. The evaluation of endometrial biopsy specimens and the classification of results are extremely important due to these problems.
An ideal classification system for diagnostic biopsies including endometrial biopsy should be biologically meaningful, predictive of the lesion, and highly reproducible among pathologists. However, the widely used WHO 1994 classification system does not fulfill all these criteria adequately.17 Therefore, more reproducible alternative classification systems have been searched in an attempt to prevent diagnostic failures and to guide clinical management. Among these, EIN system uses objective criteria which distinguish neoplastic from non-neoplastic changes.12,18-21 However, these complex and impractical criteria were successfully adopted to routine practice and Hecht et al.,13 and Mutter et al.22 showed that subjective EIN criteria reproduces and more precisely identifies endometrial precancers on hematoxylen-eosin stained sections.
In the current series, all women with EC had complex atypical EH on biopsy according to the WHO classification. Complex non-atypical or simple atypical EH was not found to be associated with EC. Therefore, neither complex architecture, nor atypical cytology alone was sufficient to consider a co-existent EC in this group of patients, but this may definitely be a result of the limited number of patients in different EH categories of the WHO classification system. On the other hand, when the EIN system was used in the same cohort, no EC was detected in women without EIN. As a result, the sensitivity and negative predictive value of complex atypical EH and EIN were 100% in predicting coexistent EC. Therefore, either of these two classification systems was highly successful for guiding the management of cases. In this context, the clinician may safely decide to manage patients conservatively without hysterectomy if the biopsy does not yield complex atypical EH or EIN. From this point of view, both classification systems appeared to be useful in terms of decreasing the rates of unnecessary surgical interventions. Nevertheless, EIN was diagnosed in more than 40% of patients with complex non-atypical EH in this study. This was associated with a lower specificity of the EIN system. Actually, similar results were obtained in another study in which 44% of patients with complex non-atypical EH and even 4% of women with simple non-atypical EH had EIN.12 Hysterectomy may be considered to be an over-treatment for these women when the operation is decided based only on presence of EIN, since no EC was detected among them. The reason for such an over-treatment may be related to term "neoplasia," which may result in more anxiety among both patients and the clinicians.
Another risk for women with EH or EIN is the possibility of progression to cancer. Women diagnosed with atypical EH are greater than 10 times more likely to develop EC compared with women diagnosed with nonatypical EH.23 On the other hand, women with EIN were reported to be 45 times more likely than patients diagnosed with a benign endometrium to progress to EC.24 A recent study by Lacey et al.25 revealed that women who were observed at least 1 year after diagnoses of EIN and atypical EH had similarly increased risk of progression to EC. However, the risk of progression to cancer could not be evaluated in this study due to the fact that all patients were treated with surgery within 2 weeks following a diagnosis of EH.
According to the results of the current study, none of the patients without EIN or complex atypical EH had co-existent cancer. Therefore, we suggest that women without EIN or complex atypical EH may be observed conservatively without hysterectomy. Again, according to our results, the risk of a co-existent EC is 26.4% in a patient with complex atypical EH and 24.3% in EIN. Therefore, if patients with complex atypical EH or EIN are subjected to hysterectomy after biopsy, nearly one fourth will be diagnosed to have a co-existent EC. This means that most of them (almost 75%) will receive surgery because of a non-malignant condition. On the other hand, having atypical EH or EIN necessitates at least hormonal treatment and close follow-up, since these lesions precede cancer by several years.13,23,24 Hence the options of surgery vs. conservative treatment may be decided after discussing these carefully with the patient and her family.
There are definitely some limitations of the current study. First of all, it was designed retrospectively. Also, the number of patients is limited. The limited number of the patients in the study did not allow the authors to compare the results according to age, preoperative risk factors, symptoms, concomitant pathologies, biopsy methods, and menopausal status. Accordingly, multivariate analysis was not possible. However, the re-evaluation of the specimens was performed by a pathologist who specializes in gynecologic pathology and was blinded to the initial pathologic results. Therefore, the diagnostic errors and recall biases were kept as minimal as possible.
In conclusion; the diagnoses of complex atypical EH and EIN had similar sensitivities with 100% negative predictive values for the prediction of coexistent EC in patients with abnormal uterine bleeding. Therefore, when a pathologist who is frequently exposed to such available specimens, either of these two classification systems may be used, and patients without EIN or complex atypical EH may safely be managed conservatively without hysterectomy. However, given the objective diagnostic criteria compared to the traditional WHO classification, the use of the objective EIN system, rather than the subjective EIN system should be preferred whenever possible to prevent diagnostic errors, and to avoid unnecessary surgical interventions in centers where an experienced pathologist is not available.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Cavanagh D, Fiorica JV, Hoffman MS, Durfee J, Nicosia SV. Adenocarcinoma of the endometrium: an institutional review. Cancer Control. 1999;6:354–360. doi: 10.1177/107327489900600405. [DOI] [PubMed] [Google Scholar]
- 3.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 4.Horn LC, Meinel A, Handzel R, Einenkel J. Histopathology of endometrial hyperplasia and endometrial carcinoma: an update. Ann Diagn Pathol. 2007;11:297–311. doi: 10.1016/j.anndiagpath.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg SG, Mutter GL, Kurman RJ, Kubik-Huch RA, Nogales F, Tavassoli FA. Tumors of the uterine corpus: epithelial tumors and related lesions. In: Tavassoli FA, Stratton MR, editors. WHO classification of tumors: pathology and genetics of tumors of the breast and female genital organs. Lyon: IARC Press; 2003. pp. 221–232. [Google Scholar]
- 6.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia: a long-term study of "untreated" hyperplasia in 170 patients. Cancer. 1985;56:403–412. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Widra EA, Dunton CJ, McHugh M, Palazzo JP. Endometrial hyperplasia and the risk of carcinoma. Int J Gynecol Cancer. 1995;5:233–235. doi: 10.1046/j.1525-1438.1995.05030233.x. [DOI] [PubMed] [Google Scholar]
- 8.Clark TJ, Neelakantan D, Gupta JK. The management of endometrial hyperplasia: an evaluation of current practice. Eur J Obstet Gynecol Reprod Biol. 2006;125:259–264. doi: 10.1016/j.ejogrb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Skov BG, Broholm H, Engel U, Franzmann MB, Nielsen AL, Lauritzen AF, et al. Comparison of the reproducibility of the WHO classifications of 1975 and 1994 of endometrial hyperplasia. Int J Gynecol Pathol. 1997;16:33–37. doi: 10.1097/00004347-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron C, Nogales FF, Masseroli M, Abeler V, Duvillard P, Muller-Holzner E, et al. A multicentric European study testing the reproducibility of the WHO classification of endometrial hyperplasia with a proposal of a simplified working classification for biopsy and curettage specimens. Am J Surg Pathol. 1999;23:1102–1108. doi: 10.1097/00000478-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Usubutun A, Ertoy D, Ozkaya O, Altinok G, Kucukali T. Search for problem areas in endometrial biopsies to achieve quality assurance. Pathol Res Pract. 2000;196:625–626. doi: 10.1016/S0344-0338(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 12.Mutter GL The Endometrial Collaborative Group. Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? Gynecol Oncol. 2000;76:287–290. doi: 10.1006/gyno.1999.5580. [DOI] [PubMed] [Google Scholar]
- 13.Hecht JL, Ince TA, Baak JP, Baker HE, Ogden MW, Mutter GL. Prediction of endometrial carcinoma by subjective endometrial intraepithelial neoplasia diagnosis. Mod Pathol. 2005;18:324–330. doi: 10.1038/modpathol.3800328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery BE, Daum GS, Dunton CJ. Endometrial hyperplasia: a review. Obstet Gynecol Surv. 2004;59:368–378. doi: 10.1097/00006254-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Gultekin M, Diribas K, Dursun P, Ayhan A. Current management of endometrial hyperplasia and endometrial intraepithelial neoplasia (EIN) Eur J Gynaecol Oncol. 2009;30:396–401. [PubMed] [Google Scholar]
- 16.Bilgin T, Ozuysal S, Ozan H, Atakan T. Coexisting endometrial cancer in patients with a preoperative diagnosis of atypical endometrial hyperplasia. J Obstet Gynaecol Res. 2004;30:205–209. doi: 10.1111/j.1447-0756.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 17.Zaino RJ, Kauderer J, Trimble CL, Silverberg SG, Curtin JP, Lim PC, et al. Reproducibility of the diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:804–811. doi: 10.1002/cncr.21649. [DOI] [PubMed] [Google Scholar]
- 18.Mutter GL, Chaponot ML, Fletcher JA. A polymerase chain reaction assay for non-random X chromosome inactivation identifies monoclonal endometrial cancers and precancers. Am J Pathol. 1995;146:501–508. [PMC free article] [PubMed] [Google Scholar]
- 19.Mutter GL, Boynton KA, Faquin WC, Ruiz RE, Jovanovic AS. Allelotype mapping of unstable microsatellites establishes direct lineage continuity between endometrial precancers and cancer. Cancer Res. 1996;56:4483–4486. [PubMed] [Google Scholar]
- 20.Jovanovic AS, Boynton KA, Mutter GL. Uteri of women with endometrial carcinoma contain a histopathological spectrum of monoclonal putative precancers, some with microsatellite instability. Cancer Res. 1996;56:1917–1921. [PubMed] [Google Scholar]
- 21.Baak JP, Mutter GL. EIN and WHO94. J Clin Pathol. 2005;58:1–6. doi: 10.1136/jcp.2004.021071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutter GL, Baak JP, Crum CP, Richart RM, Ferenczy A, Faquin WC. Endometrial precancer diagnosis by histopathology, clonal analysis, and computerized morphometry. J Pathol. 2000;190:462–469. doi: 10.1002/(SICI)1096-9896(200003)190:4<462::AID-PATH590>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 23.Lacey JV, Jr, Ioffe OB, Ronnett BM, Rush BB, Richesson DA, Chatterjee N, et al. Endometrial carcinoma risk among women diagnosed with endometrial hyperplasia: the 34-year experience in a large health plan. Br J Cancer. 2008;98:45–53. doi: 10.1038/sj.bjc.6604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baak JP, Mutter GL, Robboy S, van Diest PJ, Uyterlinde AM, Orbo A, et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer. 2005;103:2304–2312. doi: 10.1002/cncr.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacey JV, Jr, Mutter GL, Nucci MR, Ronnett BM, Ioffe OB, Rush BB, et al. Risk of subsequent endometrial carcinoma associated with endometrial intraepithelial neoplasia classification of endometrial biopsies. Cancer. 2008;113:2073–2081. doi: 10.1002/cncr.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]