Abstract
Exposure to environmental contaminants, such as polychlorinated biphenyls (PCBs), is a risk factor for the development of cardiovascular diseases such as atherosclerosis. Vascular cell adhesion molecule-1 (VCAM-1) is a critical mediator for adhesion and uptake of monocytes across the endothelium in the early stages of atherosclerosis development. The upregulation of VCAM-1 by PCBs may be dependent on functional membrane domains called caveolae. Caveolae are particularly abundant in endothelial cell membranes and involved in trafficking and signal transduction. The objective of this study was to investigate the role of caveolae in PCB-induced endothelial cell dysfunction. Primary mouse aortic endothelial cells (MAECs) isolated from caveolin-1-deficient mice and background C57BL/6 mice were treated with coplanar PCBs, such as PCB77 and PCB126. In addition, siRNA gene silencing technique was used to knockdown caveolin-1 in porcine vascular endothelial cells. In MAECs with functional caveolae, VCAM-1 protein levels were increased after exposure to both coplanar PCBs, whereas expression levels of VCAM-1 were not significantly altered in cells deficient of caveolin-1. Furthermore, PCB-induced monocyte adhesion was attenuated in caveolin-1-deficient MAECs. Similarly, siRNA silencing of caveolin-1 in porcine endothelial cells confirmed the caveolin-1-dependent VCAM-1 expression. Treatment of cells with PCB77 and PCB126 resulted in phosphorylation of extracellular signal-regulated kinase-1/2 (ERK1/2), and pharmacological inhibition of ERK1/2 diminished the observed PCB-induced increase in monocyte adhesion. These findings suggest that coplanar PCBs induce adhesion molecule expression, such as VCAM-1, in endothelial cells, and that this response is regulated by caveolin-1 and functional caveolae. Our data demonstrate a critical role of functional caveolae in the activation and dysfunction of endothelial cells by coplanar PCBs.
Keywords: PCB, caveolin-1, mouse endothelial cells, vascular inflammation
Introduction
Cardiovascular disease is the leading cause of death in the United States. A number of different factors including environmental and chemical contaminants are known contributors to cardiovascular diseases (Brook et al., 2004; Pelclova et al., 2006; Hennig et al., 2007). Extensive evidence also indicates that environmental factors increase the incidence and severity of cardiovascular diseases such as atherosclerosis. Atherosclerosis is an inflammatory disease of the arterial wall that involves dysfunction of the endothelium and vascular smooth muscle cells. This atherogenic process is initiated by accumulation of leukocytes into vascular endothelial cells from the blood circulation, and expression of adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) plays a critical role in this pathologic process. Vascular endothelial cells are exposed to the blood stream and play a key role in the regulation of macromolecule transcytosis, regulation of blood pressure and vascular tone, and homeostasis of cholesterol and lipid metabolism. Furthermore, endothelial cells are involved in signal transduction and inflammation. Thus, endothelial dysfunction is believed to result in human diseases, such as atherosclerosis. Studies have also demonstrated that endothelial dysfunction in the initiation stage of atherosclerosis can be linked to exposure to persistent organic pollutants such as polychlorinated biphenyls (PCBs) (Toborek et al., 1995; Lim et al., 2007; Arsenescu et al., 2008; Helyar et al., 2009; Majkova et al., 2009). PCBs are well-known environmental pollutants that were widely used in many industrial and commercial applications for over 50 years (ref: http://www.epa.gov/osw/hazard/tsd/pcbs/index.htm). PCBs are fat-soluble and bioaccumulate in animals and humans primarily through the food chain, inhalation and dermal contact. From epidemiological studies, there is substantial evidence that environmental pollutants such as PCBs increase the risk of developing cardiovascular disease (Ha et al., 2007; Lee et al., 2007; Everett et al., 2008), but little is known about the underlying molecular mechanisms
Caveolae are 50–100 nm flask-shaped plasma membrane invaginations that are particularly abundant in specific cell types such as endothelial cells and smooth muscle cells (Cohen et al., 2004a). Caveolae have been implicated in lipid and membrane trafficking, membrane structure and signal transduction events. The three types of caveolin proteins (caveolin-1, -2 and -3) are known to be associated with caveolae formation, but caveolin-1 (Cav-1) has been recognized as an essential component along with cholesterol and sphingolipid (Drab et al., 2001; Cohen et al., 2004a). Caveolae have been implicated in human health and diseases (Cohen et al., 2004b; Frank et al., 2009). Particularly, there is growing evidence that caveolae microdomains play a critical role in the pathology of atherosclerosis, and Cav-1 depletion can protect against the pathology of atherosclerosis (Frank et al., 2004; Li et al., 2005; Lim et al., 2007; Fernandez-Hernando et al., 2009; Majkova et al., 2009)
In the last few years, caveolae have been drawing a lot of attention with their specialized structural and functional properties. Our laboratory demonstrated that functional caveolae are required for the dysfunction of endothelial cells after exposure to PCB77 (Lim et al., 2007; Majkova et al., 2009). In the present study, we investigated the effects of coplanar PCBs, such as PCB77 and PCB126, on the expression of VCAM-1 and monocyte adhesion in endothelial cells. Furthermore, we investigated the role of functional caveolae in PCB-induced VCAM-1 expression and monocyte adhesion using mouse endothelial cells isolated from aortas of Cav-1 deficient mice.
Materials and methods
Materials
3,3’,4,4’-tetrachlorobipheny (PCB77) and 3,3’,4,4’,5-pentachlorobiphenyl (PCB126) were purchased from AccuStandard Inc. (New Haven, CT). DMSO, PD98059 and TNF-α were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies for VCAM-1 and platelet/endothelial cell adhesion molecule-1 (PECAM-1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for extracellular signal-regulated kinase-1/2 (ERK1/2), p38 MAPK and c-Jun-N-terminal kinase (JNK) were obtained from Cell Signaling Technology (Danvers, MA). Horse radish peroxidase conjugated secondary antibody was obtained from Santa Cruz Biotechnology. FITC- and Texas Red-conjugated secondary antibodies were purchased from Invitrogen (Carlsbad, CA).
Endothelial cell isolation and culture
Primary porcine endothelial cells (PECs) were used to evaluate endothelial activation and dysfunction, as well as Cav-1 siRNA gene silencing studies. Cells were isolated from porcine pulmonary arteries as described previously (Hennig et al., 1984). PECs were cultured in M199 (Gibco, Grant Island, NY), supplemented with 10% FBS (Hyclone, Logan, UT). Cav-1 siRNA gene silencing studies were confirmed using endothelial cells derived from Cav-1 deficient mice. Cav-1 deficient mice were generated in the SV129 strain and backcrossed onto a C57BL/6 background. Mouse aortic endothelial cells (MAECs) from C57BL/6 and Cav-1-deficient mice were harvested from aorta as described earlier (Srinivasan et al., 2003). Briefly, mouse aortas were excised and periadventitial fat and connective tissue were removed. Aortic rings were placed onto Matrigel (BD Biosciences, San Jose, CA) and coated with DMEM (low glucose, d-valine) supplemented with 15% heat inactivated and dialyzed FBS (Hyclone, Logan, UT), endothelial cell growth supplement (BD Biosciences, San Jose, CA), heparin (Sigma, St. Louis, MO) and penicillin/streptomycin/fungizone (Gibco, Grant Island, NY). After 5 days the aortic rings were removed, and endothelial cells were allowed to grow to confluency. Endothelial cells were detached by dispase and cultured in Type I collagen (Sigma, St. Louis, MO) coated culture vessels using DMEM supplemented with 15% heat inactivated FBS, endothelial cell growth supplement, heparin and penicillin/streptomycin. Endothelial cells were tested for purity using Dil-acetylated LDL (Biomedical Technologies, Inc., Stoughton, MA) labeling and immunocytochemical labeling of PECAM-1. Endothelial cells were used for up to passage seven. C57BL/6 and Cav-1-deficient mouse cells were used at identical passage numbers.
Endothelial cell treatments
PECs were grown to confluence and synchronized overnight in medium containing 1% FBS before initiation of cell treatments. MAECs were grown to confluence in medium containing 15% FBS followed by overnight incubation in 5% FBS containing medium until further treatment. Cells were treated with coplanar PCBs such as PCB77 and PCB126 at a concentration of 2.5 µM. Stock solutions of PCBs were prepared in DMSO. The same amounts of DMSO as in PCB-treated cells were added to control cultures. The levels of DMSO in experimental media were less than 0.05%. PCB delivery by DMSO may facilitate PCB binding to plasma proteins, such as albumin, and subsequent cell membrane transfer. Serum concentration of PCBs can reach approximately 3 µM in people exposed to these toxicants (Wassermann et al., 1979; Jensen, 1989). Therefore, cells were exposed to PCBs at a concentration of 2.5 µM in most experimental settings in the present study. Similar levels of PCBs were used in our published manuscripts (Choi et al., 2003; Eum et al., 2004; Eum et al., 2006; Eum et al., 2008). Using the MTT conversion assay, we determined that the PCBs used in the present study were not cytotoxic at the concentrations and the time intervals of the reported experiments (data not shown).
Immunofluorescence
Endothelial cells were grown to confluence on glass culture slides. Cells were washed with PBS, and then fixed with 4% formaldehyde for 10 min at room temperature. After washing with PBS, endothelial cells were incubated with 3% BSA in PBS containing 0.05% Tween-20 (PBST) for blocking non-specific binding, and subsequently incubated overnight at 4°C with PECAM-1 primary antibody (1:500). Cells were then incubated with FITC-conjugated secondary antibody (1:1000) for 1 h at room temperature. Similarly, endothelial cells were incubated with primary anti-caveolin-1 antibody (Affinity BioReagents, Golden, MO) for overnight at 4°C (1:10000). Texas Red –conjugated secondary antibody was employed to detect caveolin-1 protein expression. Cells were washed with PBS and mounted in aqueous mounting medium containing DAPI before being visualized and captured using an Olympus BX61W1 fluorescent microscope and Fluoview software.
Real-time PCR
Cells were grown in 6-well plates, and total RNA was extracted from the cells using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's protocol. Reverse transcription was performed using the AMV reverse transcription system (Promega, Madison, WI). The levels of mRNAs expression were then assessed by real-time PCR using 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) and SYBR Green master mix (Applied Biosystems). VCAM-1 mRNA levels were divided by housekeeping gene β-actin. VCAM-1 and β-actin primer sequences for SYBR Green chemistry were designed using the Primer Express Software 3.0 for real-time PCR (Applied Biosystems) and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). Porcine VCAM-1 sequences: sense, 5′-TGGAAAGACATGGCTGCCTAT-3′; antisense, 5′-ACACCACCCCAGTCACCATAT-3′; porcine β-actin sequences: sense 5′-TCATCACCATCGGCAACG-3′; antisense, 5′-TTCCTGATGTCCACGTCG-3′.
Western blotting
Whole cells were lysed in RIPA lysis buffer (Cell Signaling Technology, Danvers, MA) containing protease inhibitors (Thermo Scientific, Waltham, MA) and centrifuged at 12,000 g for 10 min at 4°C. The supernatants were stored at −80°C and the protein levels were determined by BCA assay (Pierce, Rockford, IL). Proteins were separated on 10% SDS-PAGE, transferred onto nitrocellulose membranes, blocked at room temperature with 5% non-fat milk in Tris-buffered saline containing 0.05% Tween 20 (TBST), and subsequently incubated overnight at 4°C with primary antibodies. The membranes were washed with TBST and incubated with horseradish peroxidase-conjugated secondary antibody. Primary and secondary antibody was diluted 1:1,000 in the blocking buffer. After three washes in TBST, the blots were visualized by using ECL detection reagents (Amersham Biosciences, Buckinghamshire, England) and exposure to film. Bands were quantified using UN-SCAN-IT software (Silk Scientific, Orem, UT) and normalized to β-actin or total ERK1/2 protein expression.
Monocyte adhesion
Human THP-1 monocytes were activated with TNF-α and loaded with the fluorescent probe calcein (Molecular Probes, Carlsbad, CA). Monocytes were added to endothelial cell monolayers treated with PCBs, and incubated for 30 min at 37 °C. Unbound monocytes were washed away with PBS and the monolyer was fixed with 4% formaldehyde for 30 min. Attached fluorescent monocytes were counted in randomly selected fields using a fluorescent microscope (Olympius IX70, Center Valley, PA).
Caveolin-1 siRNA and transfection
Double stranded small interfering RNA targeting caveolin-1 was synthesized as described previously (Repetto et al., 2005; Lim et al., 2007). Porcine endothelial cells were transfected with control or caveolin-1 siRNA at a final concentration of 80 nM using GeneSilencer transfection reagent (Genlantis, San Diego, CA) in OptiMEM serum free media. Cells were incubated with the transfection mixture for 4 h followed by adding FBS to achieve a final FBS concentration of 10%. After a 48 h incubation, cells were used for treatments.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) of at least three independent groups. Statistical significance was determined by one-way or two-way ANOVA followed by Student-Newman-Keuls method using Sigma Stat 3.1 software (Systat Software, San Jose, CA). A probability value p < 0.05 was considered statistically significant.
Results
Endothelial cell characterization
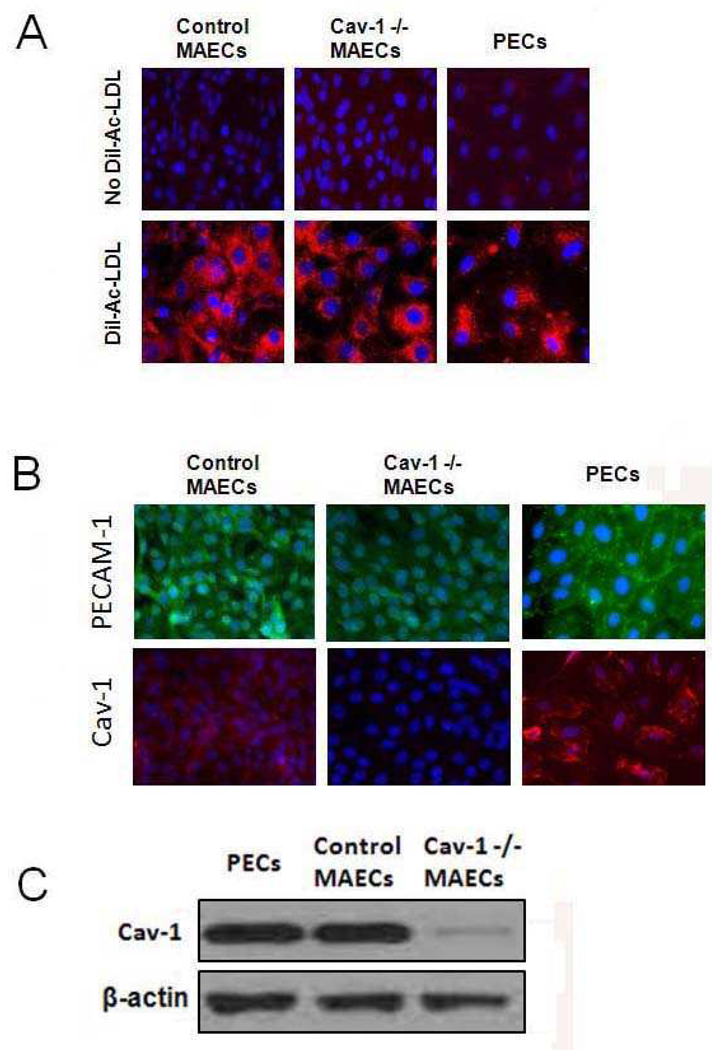
Caveolin-1 deficient (Cav-1 −/−) mice were used to isolate aortic endothelial cells. Age matched C57BL/6 mice were used as controls because the Cav-1 deficient mice are backcrossed onto C57BL/6 mice. Pictures of isolated cells were taken using conventional light microscopy, and isolated cells displayed the characteristic cobblestone morphology of endothelial cells. Endothelial cells (MAEC) were further characterized for purity and presence of the caveolin-1 gene. Dil-Ac-LDL labeling is a receptor-mediated process that is unique in endothelial cells (Netland et al., 1985). The endothelial uptake of Dil-Ac-LDL was visualized in Figure 1A. All three endothelial cell types exhibited increased uptake of oxidized LDL which is shown as red fluorescence in the cytoplasm. In addition, the endothelial cell specific marker PECAM-1 was observed in the MAECs and PECs using fluorescence microscopy (Figure 1B). Cav-1 was not detected in the Cav-1-deficient cells, but expression was observed in cells derived from wildtype mice and porcine arteries. To further identify the presence of the caveolin-1 gene, Western blotting was performed. As shown in Figure 1C, endothelial cells derived from C57BL/6 mice and pigs exhibited a large amount of caveolin-1 protein expression which was not observed in endothelial cells derived from Cav-1 −/− mice.
Figure 1.
Characterization of endothelial cells. (A) Fluorescent microscopy of mouse and porcine endothelial cells demonstrating uptake of Dil-Ac-LDL labeling. Pictures were taken at 400× magnification. (B) Fluorescent microscopy of mouse aortic endothelial cells (MAECs) and porcine endothelial cells (PECs) that express PECAM-1 (FITC, green) and caveolin-1 (Cav-1) (Texas Red, red). (C) Caveolin-1 protein expression in mouse and porcine endothelial cells determined by SDS-PAGE and Western blot analysis. β-actin was used as loading control.
Expression of VCAM-1 and adhesion of monocytes in porcine endothelial cells exposed to PCBs
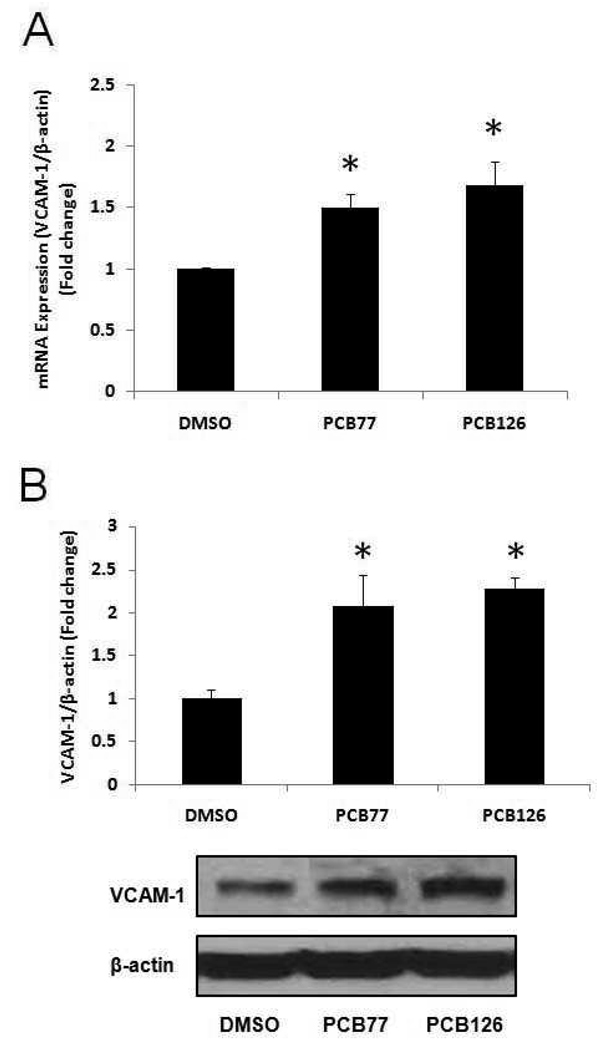
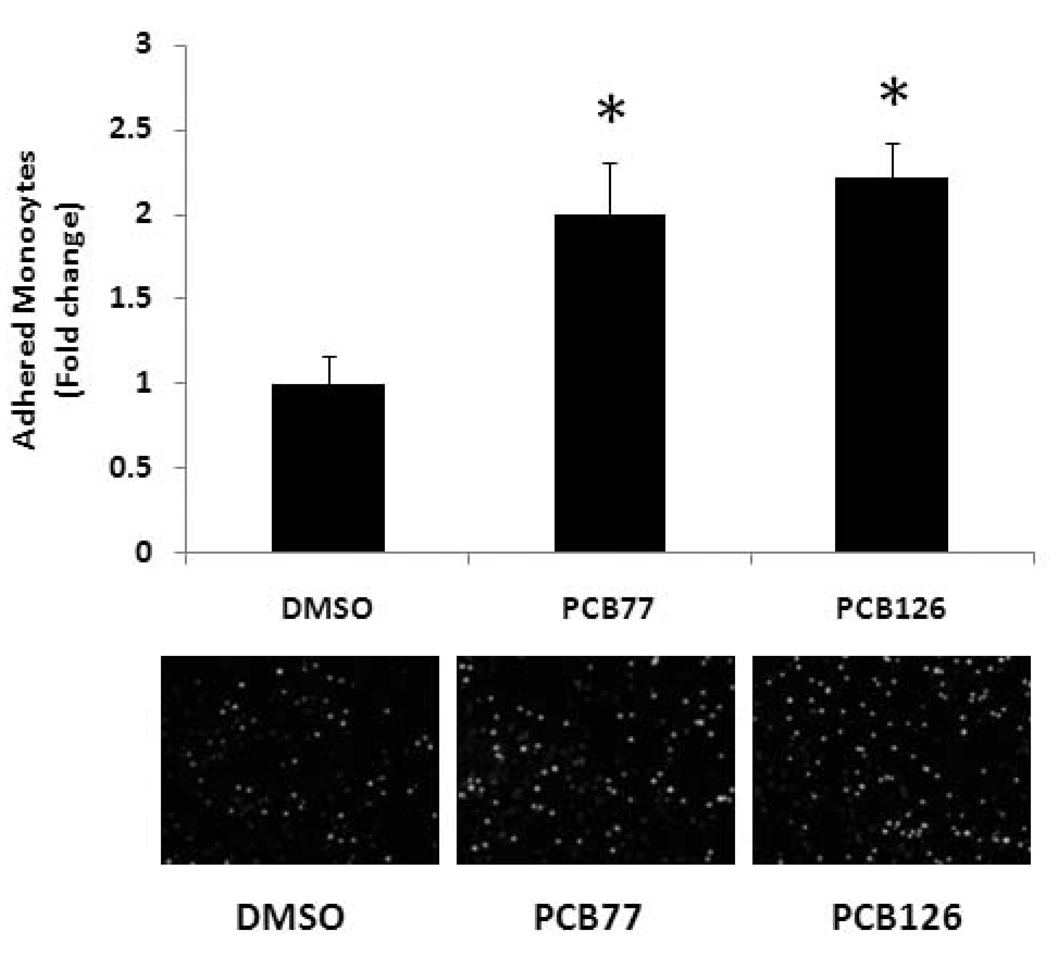
To determine whether coplanar PCBs are atherogenic in endothelial cells, we exposed both PCB77 and PCB126 to PECs at a concentration of 2.5 µM for 16 h. VCAM-1 is an immunoglobulin-like adhesion molecule expressed in endothelial cells under certain adverse physiological conditions. VCAM-1 is also known to be involved in the initial steps of monocyte recruitment to atherosclerotic lesions. Our data demonstrated a significant increase of VCAM-1 mRNA expression following exposure to both PCB77 and PCB126 (Figure 2A). VCAM-1 mRNA expression increased by approximately 50 and 68% after cell exposure to PCB77 and PCB126, respectively. The increased VCAM-1 protein expression resulting from PCB exposure was also detected by Western blot analysis (Figure 2B). Atherosclerosis is characterized by monocyte recruitment and accumulation to vascular intima. Thus, monocyte adhesion onto vascular endothelial cells is considered a critical physiological process in the pathology of atherosclerosis. In our results, both PCB77 and PCB126 significantly increased the adhesion of activated and fluorescently labeled monocytes (THP-1 cells) onto porcine endothelial cell monolayers (Figure 3). The number of attached monocytes to endothelial cells increased over 2-fold following exposure to either PCB77 or PCB126 compared to DMSO-treated control.
Figure 2.
Expression of VCAM-1 in porcine endothelial cells exposed to coplanar PCBs. (A) Expression of mRNA in porcine endothelial cells exposed to DMSO or coplanar PCBs (PCB77 and PCB126) at a concentration of 2.5 µM for 16 h was measured using real-time PCR. Results represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. (B) Expression of VCAM-1 protein after PCB treatment for 16 h. Densitometry results were normalized to β-actin. Results represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. The Western blot picture shown is a representative of three independent blots. *Significantly different compared to DMSO control.
Figure 3.
Monocyte adhesion to PECs exposed to coplanar PCBs. PECs were exposed to 2.5 µM PCB77 or PCB126 for 16 h. Human THP-1 monocytes were activated with TNF-α and loaded with the fluorescent probe calcein. Activated and calcein loaded monocytes were added to endothelial monolayers for adhesion. After washing, adhered monocytes were counted using a fluorescent microscope. Results represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. Representative microphotographs showing adhered monocytes on endothelial cell monolayers. *Significantly different compared to DMSO control.
Effect of caveolin-1 silencing on PCB-induced VCAM-1 expression and monocyte adhesion in PECs
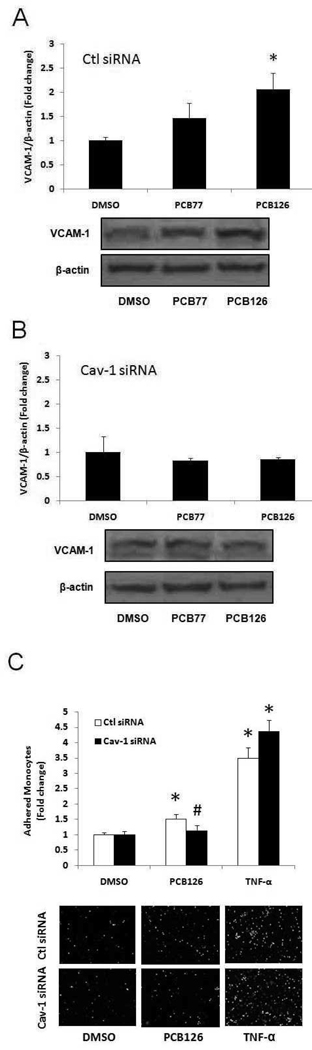
Caveolae are abundant in endothelial cells and believed to play a critical role in the pathology of atherosclerosis (Li et al., 2005). Cav-1 is the major structural protein in the formation of caveolae. In order to determine the role of Cav-1 in PCB-induced VCAM-1 expression and monocyte adhesion, we used siRNA gene silencing technique in PECs. In the control siRNA group, PCB126 significantly increased VCAM-1 protein levels, while this trend was not significant in PCB77 treated cells (Figure 4A). Cav-1 silencing significantly attenuated PCB126-induced VCAM-1 protein expression (Figure 4B). PCB77 also did not increase protein levels of VCAM-1 expression in Cav-1 siRNA group; thus, coplanar PCB-induced VCAM-1 expression appears to be Cav-1 dependent. In order to determine the role of Cav-1 on physiological endpoints, monocyte adhesion assay was performed in PECs after transfection with control or caveolin-1 siRNA. Cells were then treated with PCB126 or TNF-α for 16 h. As indicated in Figure 4C, PCB126 increased monocyte adhesion in control siRNA group, which was attenuated in Cav-1 silenced cells. TNF-α, on the other hand, significantly increased monocyte adhesion regardless of the presence or absence of functional Cav-1 in PECs.
Figure 4.
Expression of VCAM-1 in PECs lacking caveolin-1 (Cav-1). PECs treated with Cav-1 siRNA or control siRNA were exposed to PCB77 or PCB126 at 2.5 µM for 16 h. VCAM-1 protein levels in control siRNA (A) and Cav-1 siRNA (B) were observed using Western blot analysis. Densitometry results were normalized to β-actin, and represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. The Western blot picture shown is a representative of three independent blots. (C) Monocyte adhesion to control and Cav-1 siRNA tansfected PECs were exposed to PCB126 (2.5 µM, 16 h) and TNF-α (4 ng/ml, 16 h). *Significantly different compared to DMSO control. #Significantly different compared to corresponding control siRNA group.
Caveolae-dependent VCAM-1 expression and monocyte adhesion in MAECs exposed to PCBs
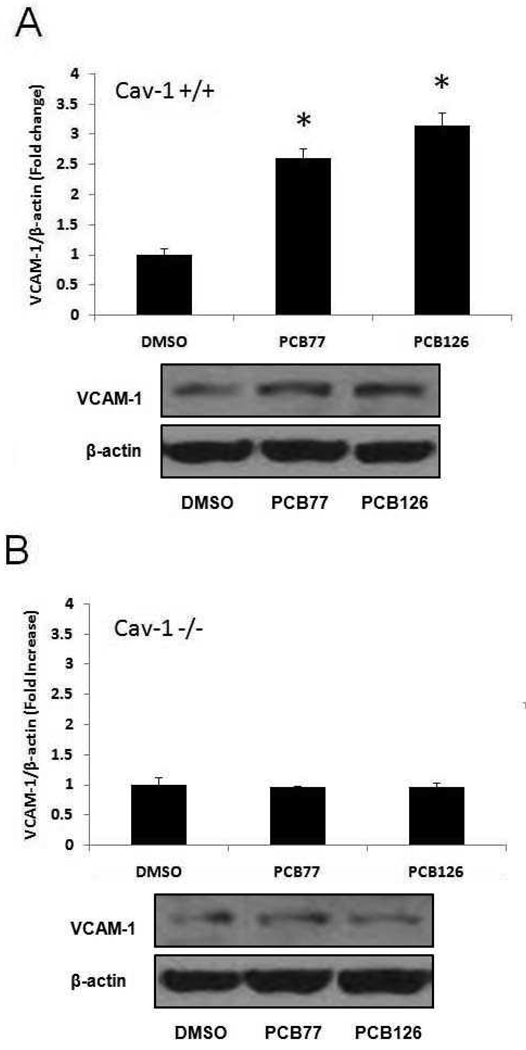
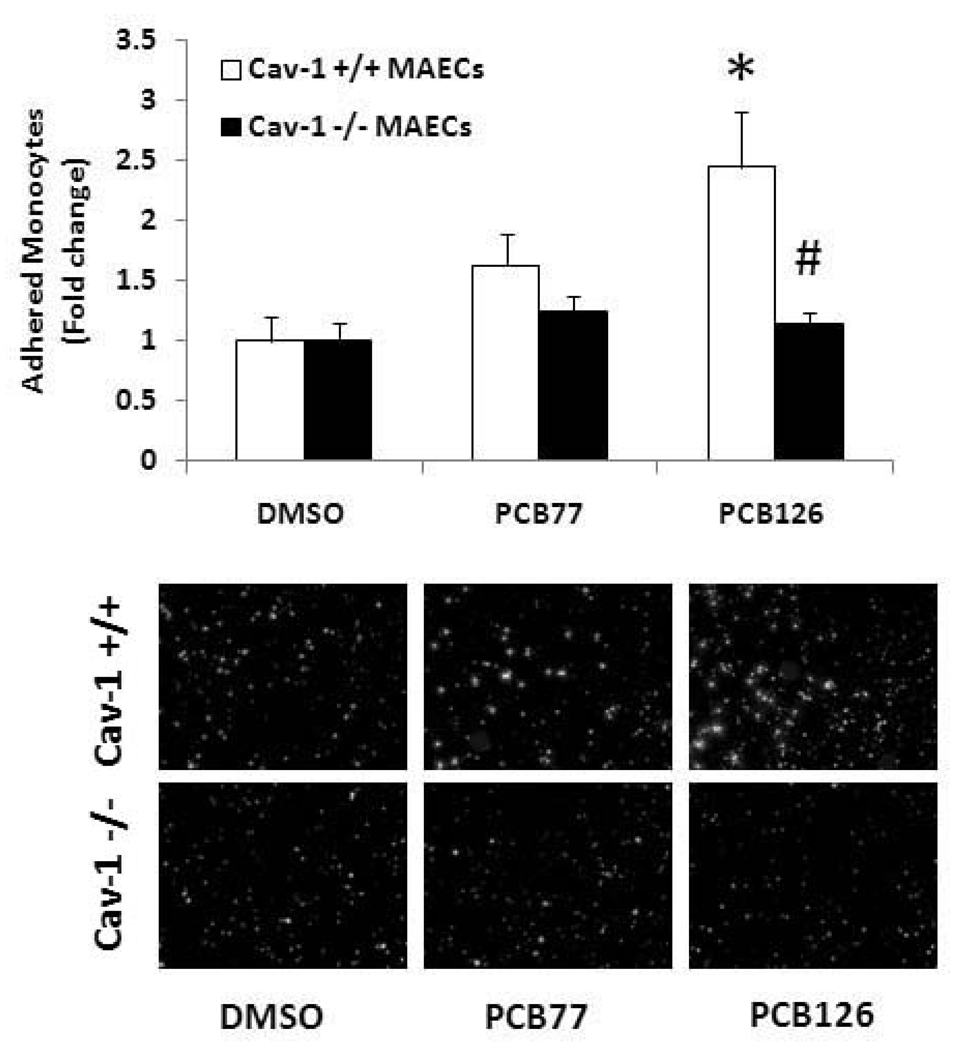
In order to further determine the role of caveolin-1 in the induction of VCAM-1 by PCB exposure, further experiments were conducted with endothelial cells derived from Cav-1-deficient mice. Age and sex matched mouse endothelial cells isolated from C57BL/6 mice were used as controls. In the Western blot analysis, both PCB77 and PCB126 significantly increased VCAM-1 protein expression in the Cav-1 containing cells derived from the wild-type mice (Figure 5A). As noted in porcine endothelial cells, the PCB-induced VCAM-1 expression was attenuated in the cells deficient in Cav-1, suggesting a critical role of Cav-1 or functional caveolae in this atherogenic pathway (Figure 5B). The increased monocyte adhesion resulting from exposure to PCB126 was significant in MAECs containing the Cav-1 gene, while this physiological response was blunted in cells lacking the Cav-1 gene (Figure 6).
Figure 5.
Expression of VCAM-1 in MAECs exposed to coplanar PCBs. Cells were exposed to DMSO or 2.5 µM PCB77 or PCB126 for 16 h, and protein levels were measured using Western blot analysis. Protein expression levels were detected in PCB treated MAECs containing caveolin-1 (A) and in caveolin-1-deficient cells (B). Densitometry results were normalized to β-actin, and represent the mean ± SEM (n=3) of three independent experiments. The Western blot picture shown is a representative of three independent blots. *Significantly different compared to DMSO control.
Figure 6.
Monocyte adhesion to MAECs exposed to coplanar PCBs. MAECs were exposed to PCB77 or PCB126 at 2.5 µM for 16 h. Human THP-1 monocytes were activated with TNF-α and loaded with the fluorescent probe calcein. Activated and calcein loaded monocytes were added to endothelial monolayers for adhesion. After washing, adhered monocytes were counted using a fluorescent microscope. Results represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. Representative microphotographs show adhered monocytes on endothelial cell monolayers. *Significantly different compared to DMSO control. #Significantly different compared to corresponding control C57BL/6 mouse endothelial cells.
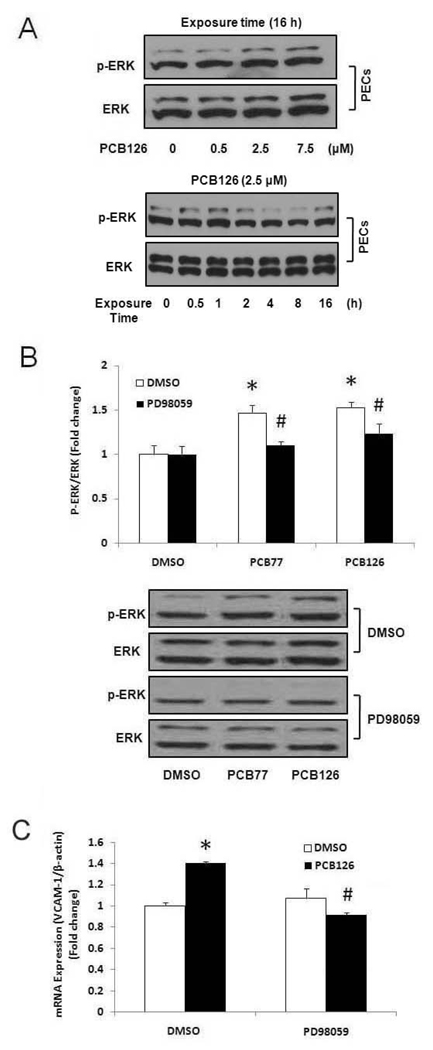
Role of extracellular signal-regulated kinase-1/2 (ERK1/2) in PCB-induced monocyte adhesion in PECs
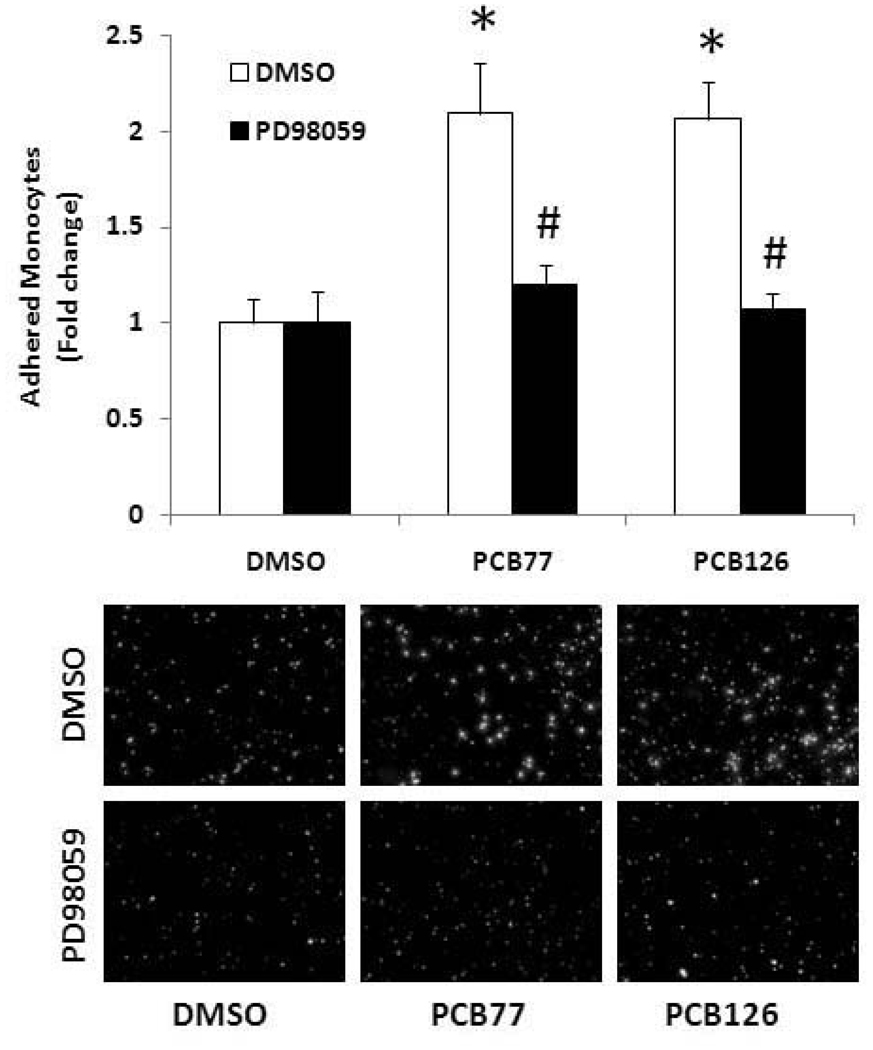
In an effort to identify signaling pathways associated with PCB-induced atherogenic effects in primary endothelial cells, we observed mitogen-activated protein kinases (MAPKs). Among the three MAPKs, ERK1/2 kinase was markedly phosphorylated after 0.5, 1 and 16 h of PCB treatment, and after 16 h of PCB exposure a dose-dependently phosphorylation of ERK1/2 kinase was observed (Figure 7A). Other MAPKs (JNK and p38) were not significantly influenced by PCB exposure (data not shown). ERK1/2 activation was attenuated by its pharmacological inhibitor PD98059 (Figure 7B). ERK1/2 was also involved in PCB-induced activation of PECs. Pretreatment with the ERK1/2 inhibitor PD98059 attenuated PCB-induced VCAM-1 mRNA expression in PECs (Figure 7C). Furthermore, the ERK1/2 inhibitor PD98059 significantly attenuated PCB-induced monocyte adhesion in PECs (Figure 8).
Figure 7.
Dose- and time-dependent phosphorylation of ERK1/2 MAPK by PCB126 in PECs (A). PECs were treated with 0–7.5 µM PCB126 for 16 h to observe a dose-dependent phosphorylation of ERK1/2. PECs were treated with 2.5 µM PCB126 for 0.5–16 h to determine time-dependent phosphorylation of ERK1/2. Western blot analysis was performed to observe phosphorylation of ERK1/2 using a monoclonal antibody. Experiments were repeated a minimum of three times. The Western blot picture shown is a representative of three independent blots. Phosphorylation of ERK1/2 MAPK by PCB77 or PCB126 in PECs (B). PECs were pretreated with 10 µM of PD98059 for 1 h followed by treatment with PCB77 or PCB126 at 2.5 µM for 16 h. Western blot analysis was performed to observe phosphorylation of ERK1/2 using a monoclonal antibody. Densitometry results were normalized to total ERK1/2, and represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. The Western blot picture shown is a representative of three independent blots. (C) Expression of VCAM-1 mRNA in porcine endothelial cells exposed to DMSO or PCB126 at a concentration of 2.5 µM for 16 h after pretreatment with PD98059. mRNA was measured using real-time PCR. Results represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. *Significantly different compared to DMSO control. #Significantly different compared to corresponding DMSO control.
Figure 8.
Monocyte adhesion to PECs after pretreatment with PD98059 followed by exposure to PCB77 or PCB126 (2.5 µM for 16 h). Human THP-1 monocytes were activated with TNF-α and loaded with the fluorescent probe calcein. Activated and calcein loaded monocytes were added to endothelial monolayers for adhesion. After washing, adherend monocytes were counted using a fluorescent microscope. Representative microphotographs show adherend monocytes on endothelial cell monolayers. *Significantly different compared to DMSO control. #Significantly different compared to corresponding control C57BL/6 mouse endothelial cells.
Association between caveolin-1 and activation of ERK1/2 in PCB-induced VCAM-1 expression
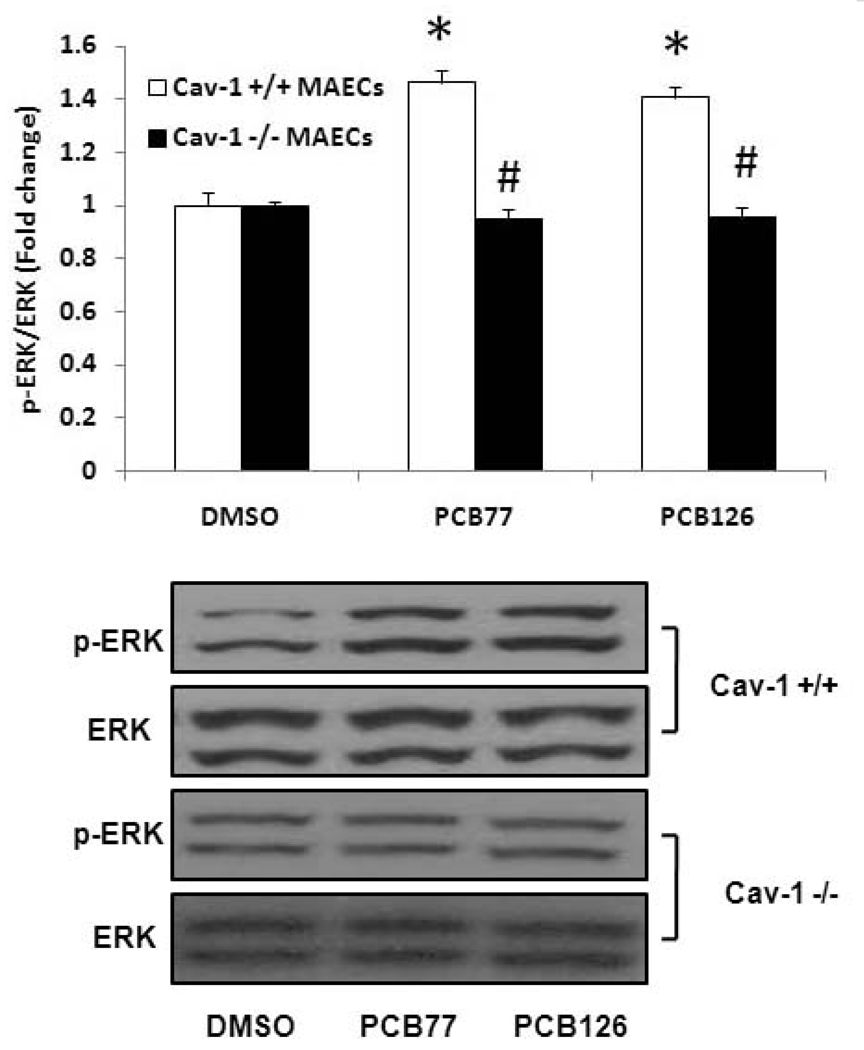
Because PCB-induced VCAM-1 expression and monocyte adhesion are found to be Cav-1 and ERK1/2-dependent events, a relationship between caveolin-1 and ERK1/2 was determined. The PCB126 induced ERK1/2 activation was found in MAECs containing Cav-1, but not significantly activated in Cav-1 deficient MAECs (Figure 9). These data suggest that PCB-induced ERK1/2 phosphorylation is Cav-1 dependent. These results imply that Cav-1 is a regulator of ERK1/2 that is responsible for upregulation of VCAM-1 and resulting adhesion of monocytes to endothelial monolayers.
Figure 9.
Phosphorylation of ERK1/2 MAPK by PCB77 and PCB126 in MAECs. MAECs were treated with PCB77 or PCB126 at 2.5 µM for 16 h. Western blot analysis was performed to observe phosphorylation of ERK1/2 using a monoclonal antibody. Densitometry results were normalized to total ERK1/2, and represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. The Western blot picture shown is a representative of three independent blots. *Significantly different compared to DMSO control. #Significantly different compared to corresponding Cav-1 +/+ C57BL/6 MAECs.
Discussion
There is epidemiological and experimental evidence linking exposure to persistent environmental pollutants to the development of cardiovascular diseases. PCBs are abundant persistent organic pollutants in the environment and are considered to be associated with the etiology of cardiovascular disease, such as atherosclerosis. For examples, an increase in cardiovascular death was observed in a high PCB exposed group among Swedish capacitor manufacturing workers (Gustavsson and Hogstedt, 1997). In addition, an increase in mortality from cardiovascular diseases was observed during 10-year follow-up studies of the Seveso dioxin pollution accident in the herbicide production factory in Italy (Pesatori et al., 1998). In animal studies, exposure to PCB126 in female rats resulted in higher cardiovascular risk factors, such as increased blood pressure, serum cholesterol and heart weight (Lind et al., 2004). A recent study also demonstrated that PCB77 may contribute to the development of obesity-associated atherosclerosis (Arsenescu et al., 2008). Moreover, studies from our laboratory demonstrated that coplanar PCBs, including PCB77 and PCB126, are proinflammatory and atherogenic in vascular endothelial cells (Hennig et al., 2002; Lim et al., 2007; Majkova et al., 2009).
Atherosclerosis is a chronic inflammatory disease of the arterial wall, and the immune system is known to be actively involved in the pathology of this disease. For example, upregulation of cell adhesion molecules and recruiting monocytes to the vessel wall are considered early events in the development of atherosclerosis (Ross, 1993; Nakashima et al., 1998; Mestas and Ley, 2008). Adhesion molecules expressed in activated endothelial cells include intercellular adhesion molecule-1, VCAM-1, P-selectin, and E-selectin, and these adhesion molecules are not routinely expressed under normal conditions. In particular, the role of VCAM-1 in early atherosclerotic lesions was investigated, and studies found that blockade of VCAM-1 in the isolated carotid artery reduced monocyte adhesion by 75% (Huo et al., 2000). Therefore, in the present study, we investigated the expression of VCAM-1 as well as monocyte adhesion in order to show proatherogenic properties of coplanar PCBs. We found that both PCB77 and PCB126 can increase mRNA and protein levels of VCAM-1, resulting in increased monocyte adhesion to endothelial cells.
Furthermore, in this report, we demonstrated that upregulation of VCAM-1and resulting monocyte adhesion in primary vascular endothelial cells in response to PCB77 and PCB126 are dependent on functional caveolae. We used two different primary vascular endothelial cells derived from pigs and mice. Most importantly, to investigate the role of caveolae, primary aortic endothelial cells used in this study were retrieved from Cav-1-deficient mice, and C57BL/6 mice were used as background control. Thus, our experiments show insights into the roles of caveolin-1 and functional caveolae during dysfunction of endothelial cells by PCB exposure. Our data demonstrated that VCAM-1 expression was significantly upregulated by coplanar PCBs in endothelial cells containing Cav-1, while this response was attenuated in cells isolated from Cav-1-deficient mice, suggesting that caveolae play an important role in PCB toxicity. Previous studies from our laboratory have shown that a single injection of PCB77 can increase VCAM-1 expression in the mouse aortas (Hennig et al., 2002). Also, the expression of eNOS and Cyp1A1 in porcine endothelial cells exposed to PCB77 was dependent on Cav-1 activity (Lim et al., 2007; Lim et al., 2008). More importantly, both MCP-1 and IL-6 were upregulated by PCB exposure only in mouse aorta containing functional Cav-1 (Majkova et al., 2009). A previous report using mice deficient of both Cav-1 and apoE also demonstrated that the loss of Cav-1 resulted in the downregulation of CD36 and VCAM-1 despite an observed hypercholesterolemia (Frank et al., 2004). In the present study, we confirm these findings in endothelial cells isolated from mice lacking Cav-1. Using siRNA gene silencing technique, function of caveolae were also confirmed in primary porcine endothelial cells, a cell culture system well characterized and routinely used in our laboratory. Our data have shown that knockdown of Cav-1 by siRNA gene silencing technique can attenuate PCB-induced VCAM-1 expression and monocyte adhesion. However, it was shown that TNF-α-induced monocyte adhesion is not associated with presence Cav-1. These data is supported by the studies demonstrating TNF-α-induced NF-κB activation is independent of Cav-1 in endothelial cells (D'Alessio et al., 2005). Taken together, our data provide strong evidence about the importance of Cav-1 and functional caveolae in the mediation of PCB toxicity in endothelial cells.
In the present study, we compared two different coplanar PCBs, i.e., PCB77 and PCB126, in the VCAM-1 expression and monocyte adhesion experiments. Both PCBs are dioxin-like congeners with high affinity to the aryl hydrocarbon receptor (AhR). AhR binding affinity and induction of cytochrome P4501A1 (Cyp1A1) are responsible for the metabolism of coplanar PCB congeners and subsequent toxic effects. Although the World Health Organization-toxic equivalency factors (WHO-TEFs) are largely different between the two PCBs (0.0001 of PCB77 vs. 0.1 of PCB126) (Van den Berg et al., 2006), our data indicate that at the same concentration (2.5 µM), both coplanar PCBs show similar proatherogenic effects in endothelial cells. This implies that additional pathways may contribute to this coplanar PCB-induced endothelial dysfunction in addition to the AhR-mediated endothelial toxic responses. Additional signaling pathways include activation of the mitogen-activated kinase (MAPK) family, which is generally believed to activate NF-κB and AP-1 and resulting in upregulation of their target genes including VCAM-1. Little is known about these intracellular signaling pathways in endothelial cells as a result of PCB exposure. Recent studies have suggested that the ERK1/2 MAPK signaling pathway is associated with Cav-1 activation in mouse embryonic stem cells stimulated by epidermal growth factor, indicating that ERK1/2 phosphorylation is dependent on the presence of Cav-1 and functional caveolae (Park and Han, 2009). Our laboratory has shown that Cav-1 siRNA can inhibit NF-κB-DNA binding activity after PCB exposure to endothelial cells (Lim et al., 2007). In addition, cell containing Cav-1 siRNA attenuated PCB-induced Cyp1A1 expression and oxidative stress (Lim et al., 2008). These data suggest that Cav-1 mediates PCB-induced endothelial cell responses where binding of PCB to AhR and resulting Cyp 1A1-induced oxidative stress may contribute to the activation of ERK1/2. Activated ERK1/2 can promote NF-κB-DNA binding and subsequently VCAM-1 expression. Our current data show that ERK1/2 MAPK can be activated by coplanar PCBs, and that this response is responsible, at least in part, for the observed monocytes adhesion to endothelial monolayers. These results were confirmed by inhibiting phosphorylation of ERK1/2 using a specific inhibitor PD98059, where PCB treatment did not increase VCAM-1 mRNA expression and monocyte adhesion. Furthermore, our studies demonstrated that PCB-induced ERK1/2 phosphorylation is dependent on the presence of Cav-1 using mouse derived Cav-1 deficient cells. Thus, our data suggest that caveolae play a role in PCB-mediated activation of the ERK1/2 MAPK signaling pathway that also upregulates monocyte-endothelium adhesion in PCB-treated endothelial cells.
In conclusion, our findings provide evidence that caveolin-1 and functional caveolae play an important role in the PCB-induced atherogenic responses in vascular endothelial calls. In addition to siRNA gene silencing, successful culture of aortic endothelial cells derived from mice deficient in Cav-1 demonstrated attenuation of PCB-mediated induction of VCAM-1 and monocyte adhesion to endothelial cells. Moreover, we found that PCB-induced atherogenic effects are also dependent, in part, on the ERK1/2 MAPK signaling cascade and that this response is a Cav-1 dependent pathway.
Acknowledgements
This research was supported by grants from NIEHS/NIH (P42ES007380) and University of Kentucky Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Choi W, Eum SY, Lee YW, Hennig B, Robertson LW, Toborek M. PCB 104-induced proinflammatory reactions in human vascular endothelial cells: relationship to cancer metastasis and atherogenesis. Toxicol Sci. 2003;75:47–56. doi: 10.1093/toxsci/kfg149. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004a;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, Brasaemle DL, Scherer PE, Lisanti MP. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004b;53:1261–1270. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- D'Alessio A, Al-Lamki RS, Bradley JR, Pober JS. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am J Pathol. 2005;166:1273–1282. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Eum SY, Andras IE, Couraud PO, Hennig B, Toborek M. Pcbs and Tight Junction Expression. Environ Toxicol Pharmacol. 2008;25:234–240. doi: 10.1016/j.etap.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum SY, Lee YW, Hennig B, Toborek M. VEGF regulates PCB 104-mediated stimulation of permeability and transmigration of breast cancer cells in human microvascular endothelial cells. Exp Cell Res. 2004;296:231–244. doi: 10.1016/j.yexcr.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Eum SY, Rha GB, Hennig B, Toborek M. c-Src is the primary signaling mediator of polychlorinated biphenyl-induced interleukin-8 expression in a human microvascular endothelial cell line. Toxicol Sci. 2006;92:311–320. doi: 10.1093/toxsci/kfj194. [DOI] [PubMed] [Google Scholar]
- Everett CJ, Mainous AG, 3rd, Frithsen IL, Player MS, Matheson EM. Association of polychlorinated biphenyls with hypertension in the 1999–2002 National Health and Nutrition Examination Survey. Environ Res. 2008;108:94–97. doi: 10.1016/j.envres.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Yu J, Suarez Y, Rahner C, Davalos A, Lasuncion MA, Sessa WC. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- Frank PG, Pavlides S, Lisanti MP. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 2009;335:41–47. doi: 10.1007/s00441-008-0659-8. [DOI] [PubMed] [Google Scholar]
- Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Am J Ind Med. 1997;32:234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999–2002. Environ Health Perspect. 2007;115:1204–1209. doi: 10.1289/ehp.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyar SG, Patel B, Headington K, El Assal M, Chatterjee PK, Pacher P, Mabley JG. PCB-induced endothelial cell dysfunction: role of poly(ADP-ribose) polymerase. Biochem Pharmacol. 2009;78:959–965. doi: 10.1016/j.bcp.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hennig B, Oesterling E, Toborek M. Environmental toxicity, nutrition, and gene interactions in the development of atherosclerosis. Nutr Metab Cardiovasc Dis. 2007;17:162–169. doi: 10.1016/j.numecd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hennig B, Shasby DM, Fulton AB, Spector AA. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984;4:489–497. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- Jensen AA. Background levels in humans. In: Kimbrough RD, Jensen AA, editors. Halogenated Biphenyls, Terphenyls, Naphthalenes, Dibenzodioxins and Related Products. In Topics in Environmental Health. Amsterdam, New York: Elsevier Science Publishers; 1989. pp. 345–380. [Google Scholar]
- Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR., Jr Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetologia. 2007;50:1841–1851. doi: 10.1007/s00125-007-0755-4. [DOI] [PubMed] [Google Scholar]
- Li XA, Everson WV, Smart EJ. Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc Med. 2005;15:92–96. doi: 10.1016/j.tcm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Lim EJ, Majkova Z, Xu S, Bachas L, Arzuaga X, Smart E, Tseng MT, Toborek M, Hennig B. Coplanar polychlorinated biphenyl-induced CYP1A1 is regulated through caveolae signaling in vascular endothelial cells. Chem Biol Interact. 2008;176:71–78. doi: 10.1016/j.cbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H3340–H3347. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- Lind PM, Orberg J, Edlund UB, Sjoblom L, Lind L. The dioxin-like pollutant PCB 126 (3,3',4,4',5-pentachlorobiphenyl) affects risk factors for cardiovascular disease in female rats. Toxicol Lett. 2004;150:293–299. doi: 10.1016/j.toxlet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Majkova Z, Smart E, Toborek M, Hennig B. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicol Appl Pharmacol. 2009;237:1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- Netland PA, Zetter BR, Via DP, Voyta JC. In situ labelling of vascular endothelium with fluorescent acetylated low density lipoprotein. Histochem J. 1985;17:1309–1320. doi: 10.1007/BF01002528. [DOI] [PubMed] [Google Scholar]
- Park JH, Han HJ. Caveolin-1 plays important role in EGF-induced migration and proliferation of mouse embryonic stem cells: involvement of PI3K/Akt and ERK. Am J Physiol Cell Physiol. 2009;297:C935–C944. doi: 10.1152/ajpcell.00121.2009. [DOI] [PubMed] [Google Scholar]
- Pelclova D, Urban P, Preiss J, Lukas E, Fenclova Z, Navratil T, Dubska Z, Senholdova Z. Adverse health effects in humans exposed to 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD) Rev Environ Health. 2006;21:119–138. doi: 10.1515/reveh.2006.21.2.119. [DOI] [PubMed] [Google Scholar]
- Pesatori AC, Zocchetti C, Guercilena S, Consonni D, Turrini D, Bertazzi PA. Dioxin exposure and non-malignant health effects: a mortality study. Occup Environ Med. 1998;55:126–131. doi: 10.1136/oem.55.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto S, Salani B, Maggi D, Cordera R. Insulin and IGF-I phosphorylate eNOS in HUVECs by a caveolin-1 dependent mechanism. Biochem Biophys Res Commun. 2005;337:849–852. doi: 10.1016/j.bbrc.2005.09.125. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Yeh M, Danziger EC, Hatley ME, Riggan AE, Leitinger N, Berliner JA, Hedrick CC. Glucose regulates monocyte adhesion through endothelial production of interleukin-8. Circ Res. 2003;92:371–377. doi: 10.1161/01.RES.0000061714.74668.5C. [DOI] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, Espandiari P, Robertson LW, Hennig B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. J Biochem Toxicol. 1995;10:219–226. doi: 10.1002/jbt.2570100406. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann M, Wassermann D, Cucos S, Miller HJ. World PCBs map: storage and effects in man and his biologic environment in the 1970s. Ann N Y Acad Sci. 1979;320:69–124. doi: 10.1111/j.1749-6632.1979.tb13137.x. [DOI] [PubMed] [Google Scholar]