Table 2.
Representative components and products synthesized using Bohdan MiniBlock platform
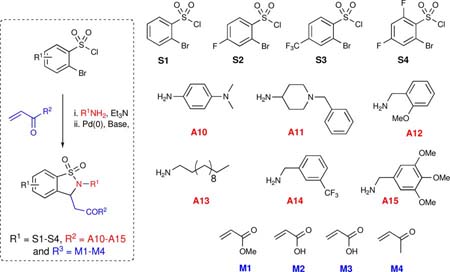
| entry | R1 | R2NH2 | R3 | yield (%) | purity (%) | product |
|---|---|---|---|---|---|---|
| 1 | S1 | A10 | M1 | 92 | 98 | 57 |
| 2 | S1 | A11 | M4 | 12 | 98 | 58 |
| 3 | S1 | A12 | M3 | 44 | 100 | 59 |
| 4 | S1 | A13 | M4 | 6 | 100 | 60 |
| 5 | S1 | A14 | M2 | 56 | 100 | 61 |
| 6 | S1 | A15 | M2 | 68 | 100 | 62 |
| 7 | S2 | A10 | M1 | 93 | 99 | 63 |
| 8 | S2 | A10 | M4 | 82 | 99 | 64 |
| 9 | S2 | A11 | M3 | 16 | 100 | 65 |
| 10 | S2 | A12 | M1 | 84 | 90 | 66 |
| 11 | S2 | A12 | M3 | 16 | 97 | 67 |
| 12 | S2 | A15 | M2 | 82 | 100 | 68 |
| 13 | S3 | A10 | M1 | 40 | 100 | 69 |
| 14 | S3 | A10 | M3 | 32 | 95 | 70 |
| 15 | S3 | A10 | M4 | 52 | 100 | 71 |
| 16 | S3 | A11 | M3 | 18 | 91 | 72 |
| 17 | S3 | A12 | M1 | 36 | 92 | 73 |
| 18 | S3 | A12 | M4 | 20 | 100 | 74 |
| 19 | S3 | A13 | M1 | 30 | 100 | 75 |
| 20 | S3 | A13 | M3 | 34 | 93 | 76 |
| 21 | S3 | A13 | M4 | 16 | 100 | 77 |
| 22 | S4 | A10 | M1 | 32 | 100 | 78 |
| 23 | S4 | A11 | M1 | 30 | 99 | 79 |
| 24 | S4 | A11 | M3 | 24 | 97 | 80 |
| 22 | S4 | A12 | M3 | 8 | 100 | 81 |
| 23 | S4 | A13 | M1 | 22 | 100 | 82 |
| 24 | S4 | A13 | M4 | 16 | 100 | 83 |
a Reaction conditions: 1 (0.136 mmol), Pd2(dba)3.CHCl3 (2 mol %), Michael acceptor (0.40 mmol), Bu4NCl (0.136 mmol) in DMF at 110 °C for 14 h. b Purified by an automated preparative reverse phase HPLC (detected by mass spectroscopy). c Purity was determined by HPLC with peak area (UV) at 214 nm.
