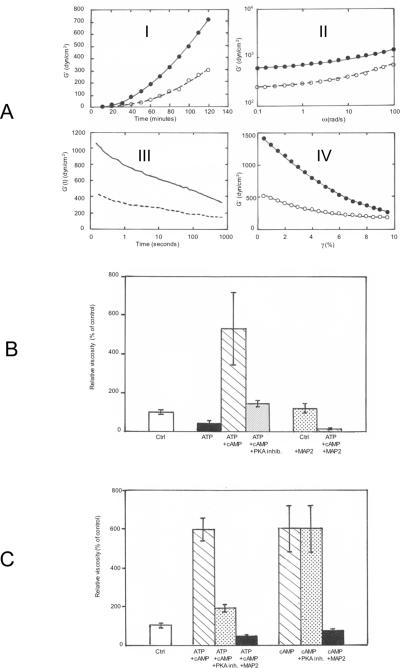
Figure 2.
A: Rheological study of MT gelation. Stimulation by cAMP + ATP
2× MTs (6 mg/ml) in buffer B, were incubated in a strain-controlled rheometer at 35 °C in the presence (closed symbols and plain line) or the absence (open symbols and dotted line) of 5 μM cAMP and 100 μM ATP.
I: Shear storage modulus (G') of MT suspensions during the polymerization initiated by bringing the sample from 4 °C in ice to 35 °C in the rheometer. The values of G' increases progressively after 30 minutes in both samples.
II: Mild increase in G' measured in the fully polymerized samples (after 2 h incubation at 35 °C for samples in I) at increasing oscillation frequencies of the rheometer, indicative of the gel state of the suspension.
III: MT gels were submitted to a small strain (2%) at T = 0 (same samples as in II). The static shear modulus G' was recorded during the relaxation of samples following the constant strain. The time dependent linear decrease in G shows that MT gels are fragile and reorganize after the application of a mild strain.
IV: G' measured in MT gels submitted to increasing strain does not exhibit strain-hardening. The strain-dependent lower elasticity suggests instead that the gel breaks easily.
B: Regulation of MT viscosity by the phosphorylation of MAPs via the cAMP-dependent MAP2-bound PKA
Samples of 2× MTs (6 mg/ml) in buffer B were mixed at 4 °C with or without 100 μM ATP +/− 5 μM cAMP, in combination or not with 0.25 μM PKA thermostable inhibitor or 50 μg thermostable MAP2, as indicated. The viscosity of samples was measured on triplicate samples after 12 h incubation at 35 °C in sealed capillaries. Average values +/− s.d. are expressed as the percentage of control MTs (no addition).
C: Comparison of the effects on MT gelation of the activation of the MAP2-bound PKA activity by cAMP with that of cAMP alone in the absence of ATP
Samples of 2× MTs (6 mg/ml) in buffer B were mixed at 4 °C with 100 μM ATP + 5 μM cAMP, in combination or not with 0.25 μM PKA thermostable inhibitor or 50 μg thermostable MAP2, as indicated. In a parallel set of assays, the same conditions were adopted but without adding ATP. The viscosity of samples was measured on triplicate samples after 12 h incubation at 35 °C in sealed capillaries. Average values +/− s.d. are expressed as percentage of control MTs (no addition).