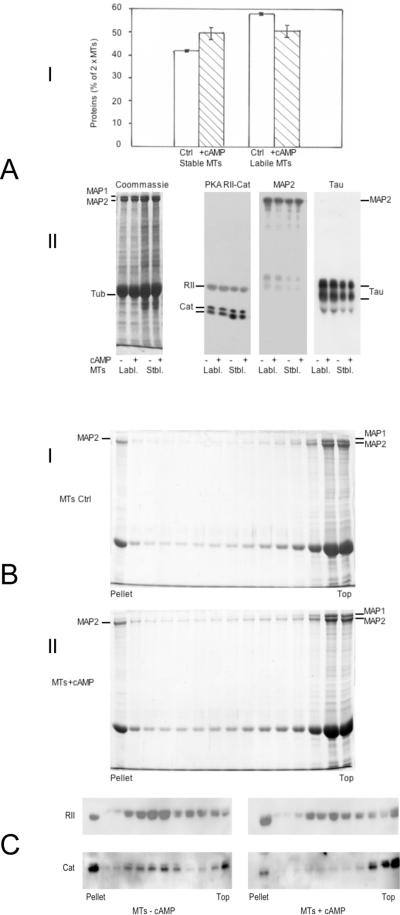
Figure 3.
A: Effect of 5 μM cAMP on the repartition of MT proteins during a polymerization cycle
1× MTs (6 mg/ml) in buffer B were repolymerized for 45 minutes at 35 °C in the absence or the presence of 5 μM cAMP (no ATP). MT pellets recovered by centrifugation 1 h at 48 000 × g at 35 °C were resuspended at 4 °C in 1/5th of initial volume of buffer B +/− 5 μM cAMP for depolymerization. After centrifugation at 100 000 × g for 40 minutes, the total amount of proteins in supernatants (2× cold-labile MTs) and pellets (2× cold-stable MTs) were measured.
I: The repartition of MT proteins into cold-stable and cold-labile fractions after a second polymerization cycle is expressed as the % of total initial amount of MTs 1×. The mean values +/− s.d. of two separate experiments are shown.
II: 7.5% acrylamide SDS-PAGE and immunoblotting of identical amounts of cold-labile (7.5 μg) and cold-stable (12 μg) MTs obtained from the second polymerization cycle +/− cAMP. The samples were probed successively with antibodies against the RII and catalytic subunits of MAP2-bound PKA, and antibodies against MAP2, and Tau. Left panel: Coommassie blue staining of the same MT proteins (40 μg).
B: Selective aggregation of MAP2-containing MTs during MT gelation
2× MTs (3 mg/ml) in buffer B containing 7.5% sucrose were mixed at 4 °C +/− 5 μM cAMP and incubated at 35 °C on top of a linear 15%–60% sucrose gradient in the same buffer. After 2 h, the gradients tubes were centrifuged for 30 minutes at 35 000 × g and 35 °C, 0.8 ml fractions were collected and 25 μl aliquots were analyzed on 7.5% acrylamide SDS-PAGE, stained with Coomassie blue. I: Control MTs: 36.6% of total MT proteins enter the gradient (pellet + fractions 1–9 / total MTs loaded). II: MTs + 5 μM cAMP: 44.2% of total MT proteins enter the gradient (pellet + fractions 1–9 / total MTs loaded).
C: cAMP induces the segregation of RII and Catalytic subunits of the PKA on distinct MT species during MT gelation
2× MTs (4 mg/ml) in buffer B containing 7.5% sucrose were incubated +/− cAMP at 35°C and centrifuged on top of a linear 15%–60% sucrose gradient in the same conditions as in figure 3B. 25 μl aliquots of all fractions were analyzed by SDS-PAGE (7.5% acrylamide) and immunoblotting for the detection of the PKA subunits by specific antibodies.