Table 2.
NHC-catalyzed cyclization-lactonization of substrate 13.
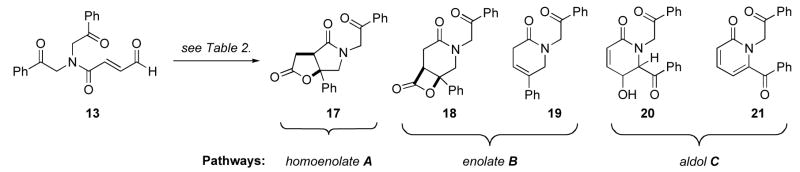 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst (mol%) | Base (mol%) | Solvent (conc.) | Temp. (°C) | A | : | B | : | Ca |
| 1 | RMesCl (15%) | DBU(10%) | tBuOH (0.1M) | 60 | 3 | : | 1 | : | 2 |
| 2 | IMesCl (15%) | DBU(10%) | tBuOH (0.1M) | 60 | 1 | : | 1.5 | : | 8 |
| 3 | 12 (15%) | DBU(10%) | tBuOH (0.1M) | 60 | 4 | : | 1 | : | 1 |
| 4 | RMesCl (15%) | DBU(10%) | 10:1 THF:tBuOH (0.10 M) | 60 | 1 | : | 1.5 | : | - |
| 5 | RMesCl (15%) | DBU(10%) | 10:1 THF:tBuOH (0.10 M) | 40 | 1 | : | 1.3 | : | - |
| 6 | RMesCl (15%) | DIPEA(10%) | 10:1 THF:tBuOH (0.10 M) | 40 | 1 | : | 1.2 | : | - |
| 7 | RMesCl (15%) | tBuOK(10%) | 10:1 THF:tBuOH (0.10 M) | 40 | 1 | : | 1 | : | 1 |
| 8 | RMesCl (15%) | DBU(10%) | 10:1 THF:tBuOH (0.10 M) | 20 | 1 | : | - | : | 6.7 |
| 9 | RMesCl (15%) | DBU(10%) | 10:1 THF:tBuOH (0.05 M) | 40 | 4 | : | 1 | : | 1 |
| 10 | RMesCl (15%) | DIPEA(10%) | 10:1 THF:tBuOH (0.05 M) | 40 | 1 | : | 1 | : | trace |
| 11 | RMesCl (15%) | DBU (50%) | 10:1 THF:tBuOH (0.05 M) | 40 | 1 | : | - | : | 10 |
| 12 | RMesCl (15%) | DIPEA (50%) | 10:1 THF:tBuOH (0.05 M) | 40 | 1 | : | 1 | : | - |
| 13 | RMesCl (15%) | DBU (10%) | 10:1 THF:tBuOH (0.01 M) | 40 | 3 | : | 1 | : | trace |
All reactions listed proceeded with 100% conversion; isolated yields were not determined.
Product pathway ratios determined from 1H NMR analysis of umpurified reaction mixtures.
