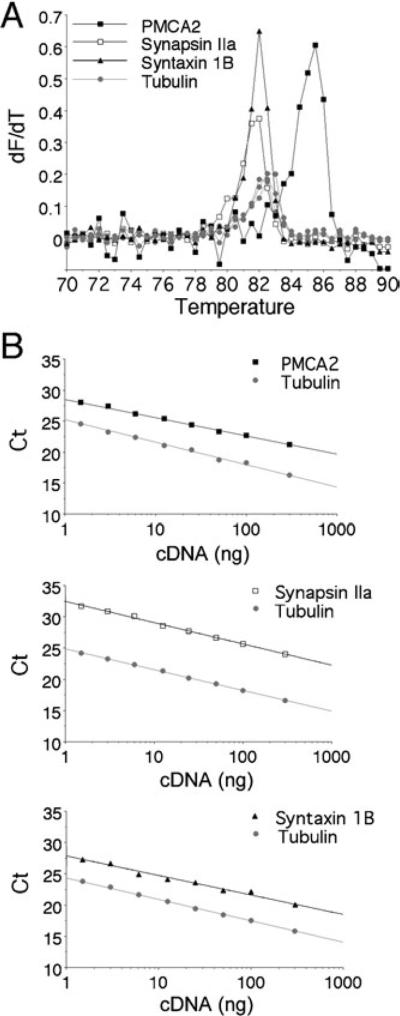
Fig. 1.
Assessment of real-time quantitative multiplex PCR using LUX fluorogenic primers. Complementary DNA template synthesized from spinal cord mRNA was analysed for target (PMCA2; synapsin IIa or syntaxin 1B) transcript levels using FAM-labelled specific primers and simultaneously for α-tubulin transcript level using a JOE-labelled primer. (A) Representative melting curves [temperature vs. fluorescence (dF/dT)]. The uniqueness of the amplicon synthesized after PCR using the FAM-labelled target primers and the JOE-labelled alpha-tubulin primer were assessed by melting analysis. Ramping of the temperature to 90 °C produced a single unique DNA dissociation curve at 82–85 °C for each fluorescence channel. (B) Real-time PCR standard curves. Wells containing serial dilutions of total cDNA template (300–1.5 ng) were assayed to generate a standard curve for target and alpha-tubulin transcripts. The Ct for each dilution is determined and expressed as a function of total cDNA loaded. The standard curves demonstrate a wide dynamic range that was linear over at least two orders of magnitude. The linear regression correlation coefficient for each curve is > 0.98, and the slope (indicating efficiency) approximates 3.1 for PMCA2, 3.4 for synapsin IIa, 3.2 for syntaxin 1B and 3.3–3.4 for α-tubulin. As the slopes for target and α-tubulin transcripts were similar (difference < 0.4), transcript relative levels in experimental samples were further calculated from the respective Ct values for target gene and α-tubulin using the 2-ΔΔCt method.