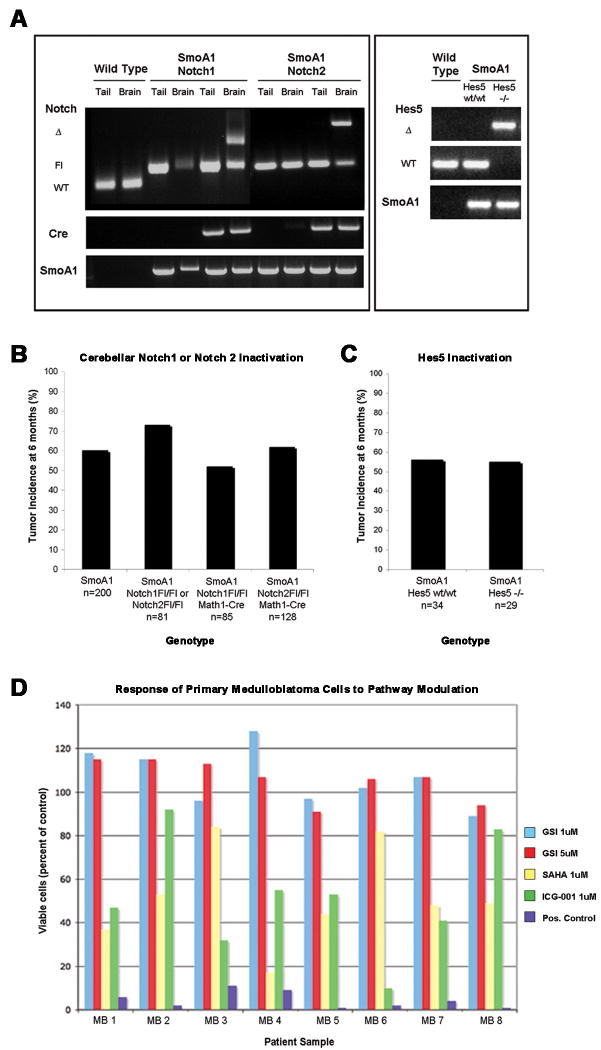
Figure 4. Conditional inactivation of Notch1, Notch2 or Hes5 in the cerebellum does not affect the incidence of medulloblastoma in SmoA1 mice.
(4a) Genomic DNA was isolated from both the tail and from whole cerebellar extracts from wild type mice and from SmoA1, Notch1Fl/Fl and SmoA1, Notch2Fl/Fl animals (Cre positive and Cre negative) and genotyping carried out as previously described (Hallahan et al., 2004, McCright et al., 2006; Radtke et al., 1999). Genotyping primers distinguished between the endogenous (WT), floxed (Fl) and null (Δ) Notch alleles, and the null alleles were present only within the cerebellum of SmoA1, Cre+, Notch1Fl/Fl and SmoA1, Cre+, Notch2Fl/Fl animals, and not in the tail DNA samples. The Hes5 deletion was not conditional, and thus representative genotyping results are shown for genomic DNA isolated from toe snips of wild type mice and from SmoA1, Hes5wt/wt and SmoA1, Hes5-/- mice. (4b) There was no significant difference in medulloblastoma incidence when the SmoA1 transgene was expressed in the absence of Notch1 (52%, n=85, p=0.06) or Notch2 (62%, n=128, p=0.66) compared to mice that expressed both copies of all Notch genes (64%, n=281 (combined columns 1 and 2)). (4c) There was no significant difference in medulloblastoma incidence between SmoA1 that were wild type (56%, n=34) versus null for Hes5 (55%, n=29, p=1.0). The study in Figure 4c was designed to detect a 50% decrease in tumor incidence with greater than 95% power (2-sided binomial test with alpha=0.05); and this power was increased for the SmoA1, Notch1 and SmoA1, Notch2 compound mutant mice with greater than 95% power to detect a 35% decrease in tumor incidence (2-sided, with alpha=0.05). All p values were generated using Fisher's exact test. SmoA1 mice lacking Hes5 were created by crossing SmoA1 mice with Hes5 null mice until homozygous deletion of Hes5 was achieved (Cau et al., 2000; Hallahan et al., 2004). SmoA1 mice lacking cerebellar Notch1 or Notch2 were generated by Cre-lox inactivation via crosses of Smo/Smo, Notch1Fl/Fl, Notch2Fl/Fl, and MATH1-Cre mice using a complex breeding strategy, which is summarized in Supplementary Figure 2. (4c) Response of primary human medulloblastoma cultures to inhibition of notch signaling by MRK-003 (GSI); the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA; vorinostat); the Wnt pathway inhibitor ICG-001; or a positive control of 10uM cisplatin, 10uM etoposide and 25uM cyclosporin A. Surgical specimens from children with medulloblastomas were incubated with drugs at the designated final concentration for 72 hours and assayed by the ViaLyte cell viability assay. All data is compared to cells grown in 0.1% DMSO, which is the vehicle used in all other conditions.