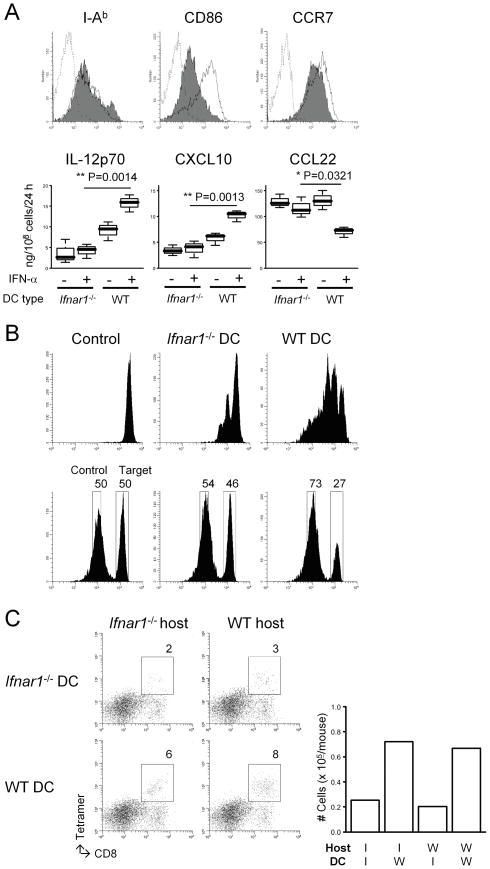
Figure 3. Ifnar1−/− DCs demonstrate defects in their APC function and the ability to attract CTLs in the glioma sites.
(A) Bone marrow-derived mature DCs were prepared as described in Materials and Methods. (Upper panels) Flow cytometry was performed to evaluate the following surface markers: I-Ab (an MHC class II), CD86, and CCR7. Histograms represent the following DC types: Ifnar1−/− (shaded), WT (open), and WT DCs stained with isotype control IgG (dashed line). (Lower panels) Immature DCs were matured with 250 ng/ml LPS in the presence or absence of 10 ng/ml rmIFN-α. ELISA was performed to evaluate the production levels of the following molecules per the last 24 hours: mIL-12p70, mCXCL10, and mCCL22. (B) WT mice received s.c. immunization with either Ifnar1−/− or WT DCs loaded with 5 μg/ml hgp10025–33 (upper) or Garc177–85 (lower) peptide on Day 0. Control mice received PBS. (Upper panels) On day -1, mice received an i.v. injection of 5 × 106 naive Pmel-I CD8+ T cells labeled with CFSE. On day 6, inguinal LN cells were collected to evaluate the proliferation of CSFE-labeled gp100-reactive cells by flow cytometry. (Lower panels) On day 6, the Garc177–85-specific cytolytic ability of effector T cells was evaluated by in vivo cytolytic assay as described in Materials and Methods. (C) Mice with developing gliomas received intratumoral injections of DCs derived from Ifnar1−/− or WT mice (1 × 105/mouse) on day 45 after birth, and i.v. Tc1 infusions on the same day (3 × 106/mouse). Mice were then sacrificed on day 5 following the DC injection for flow-cytometric evaluation of Tc1 infiltration into the tumor sites. Numbers in dot plots indicate percentage of CD8+gp100-teramer+ cells in lymphocyte-gated BILs. Absolute numbers are depicted in the rightmost panel.