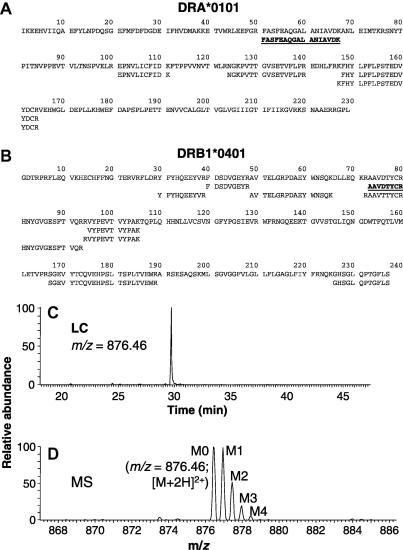
Fig. 2.
Identification of peptides suitable for 2H2O labeling studies. (A and B) Identification of Ala-/Gly-containing HLA-DR4-derived tryptic peptides. DRα (A) and DRβ (B) bands excised from SDS gels of L243 immunoprecipitates from Priess cell extracts were reduced, carbamidomethylated, digested with trypsin, and analyzed by LC–MS/MS. Alignments of tryptic fragments to the sequence of their parent polypeptides are shown. Peptides selected for analysis are shown in bold type and underlined. (C and D) LC–MS analysis of mass isotopomer distributions for the DRα peptide, FASFEAQGALANIAVDK. (C) LC chromatogram of a DRα digest, with SIM for m/z = 876.45, corresponding to the M0 mass isotopomer of the doubly charged peptide. Integration of the principal LC peak for this mass isotopomer, and for higher order mass isotopomers, was used to quantify mass isotopomer distributions. (D) Mass spectrum for the LC peak of the intact DRα peptide showing a single, well-resolved set of mass isotopomers.