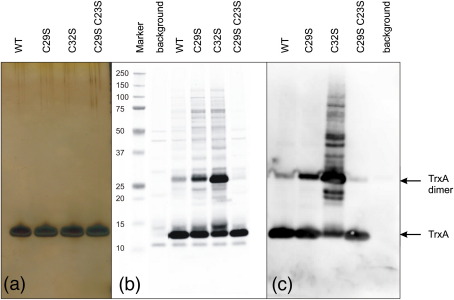
Fig. 1.
Mixed disulfide fishing. Mixed disulfide fishing was performed with the highly pure His6-tagged BsTrxA with the wild-type active site or with active-site-specific mutations, and cytoplasmic proteins from the TrxA-depleted B. subtilis WB800 ItrxA strain. (a) To show that the BsTrxA proteins used for mixed disulfide fishing were highly pure, 0.1 μg of each BsTrxA protein variant was loaded on SDS-PAGE. Upon electrophoresis, the gel was silver-stained. WT, BsTrxA with wild-type active site; C29S, C29S single-mutant BsTrxA; C32S, C32S single-mutant BsTrxA; C29S–C33S, C29S–C32S double-mutant BsTrxA. (b) To visualize possible stable interactions between BsTrxA and its substrates, 2 μg of each BsTrxA protein variant was mixed with 50 μl of cytoplasmic protein extract. After 5 min of incubation, proteins were separated on a nonreducing gel, and BsTrxA–substrate complexes were visualized by Western blotting, with antibodies raised against B. subtilis TrxA. Background: The cytoplasmic extract mock-treated with 2 μl of water instead of BsTrxA protein reveals a few nonspecific cross-reactions of the BsTrxA antibody. (c) Immunodetection of purified BsTrxA–substrate complexes. For purification of the BsTrxA–substrate bound complexes, the C-terminal His6-tag of the pure BsTrxA proteins was used. Pure BsTrxA with the wild type or a mutant active site was mixed with cytoplasmic protein extracts as indicated for (b). Subsequently, magnetic beads precharged with nickel were added, and the His6-tag of the BsTrxA proteins was allowed to bind to the nickel of the magnetic beads for 10 min. A magnet was then used to collect the beads with bound BsTrxA. After the beads had been washed nine times, the BsTrxA proteins were eluted from the beads with a buffer containing imidazole. The eluted proteins were separated by nonreducing SDS-PAGE, and the BsTrxA–substrate bound complexes were visualized by Western blotting with antibodies against BsTrxA.