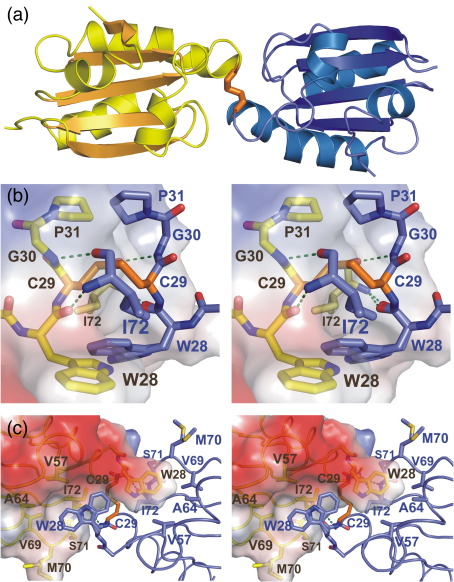
Fig. 3.
Overall fold and the dimer–interface interactions of the C32S BsTrxA dimer. (a) Overall fold of the C32S BsTrxA dimer. The disulfide-bonded C32S BsTrxA chains are shown in yellow (chain A) and in blue (chain B). The C29–C29 disulfide bond between the chains is shown in orange. (b) Stereo diagram of the active site of the C32S BsTrxA dimer with an electrostatic surface shown for chain A. The C32S active-site residues Trp28, Cys29, Gly30, and Pro31 are shown. Carbon atoms are shown in yellow in chain A and in blue in chain B. Nitrogen and oxygen atoms are in dark blue and red, respectively. The disulfide bond atoms are in orange, and hydrogen bonds are shown in green. (c) The shallow hydrophobic binding sites for the Trp28 residues on the opposite chains of the dimer in the C32S mutant of BsTrxA. The color coding is the same as in (b), and the view is an approximately 90° rotation relative to (b).