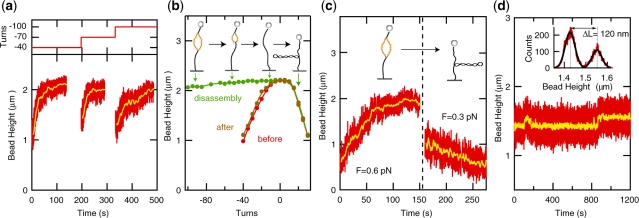
Figure 4.
Mechanochemical regulation of the reaction kinetics. (a) Binding curves obtained after application of an increasing number of unwinding turns (n=–40, −70 and −100). Reactions are reinitiated and binding of hRPA to an extended stretch of DNA occurs after each increase in the number of negative turns. (b) Dissociation induced by application of positive (rewinding) coiling (from n=−100 to n=+30). The length of the molecule is recorded in five turn intervals. The resulting rotation curve is displayed in Figure 4b (green). The long plateau indicates that protein dissociation is induced by positive coiling. The rotation curve taken after dissociation (brown) closely matches the rotation curve measured for the bare DNA molecule prior to the experiment (red) (F=0.55 pN, [NaCl]=30 mM). (c) Shift of reaction equilibrium by a modest change in force. At F=0.6 pN, hRPA association is observed ([hRPA]=100 nM). At 0.3 pN, rapid dissociation occurs for the exact same buffer condition ([NaCl]=120 mM). (d) hRPA-induced dsDNA unwinding under conditions where kon ≈ koff, [NaCl]=150 mM, F=0.8 pN, [hRPA]=120 nM, n=−30. The end-to-end-distance of the molecule fluctuates between two stable levels indicative of an individual molecule hopping on and off the dsDNA substrate. Change in length, ΔL=120 nm (see histogram of filtered extension data in inset), corresponds to the removal of 3.1±0.1 supercoils during the binding event, indicative of an hRPA occluded binding size of 31±1 nt.