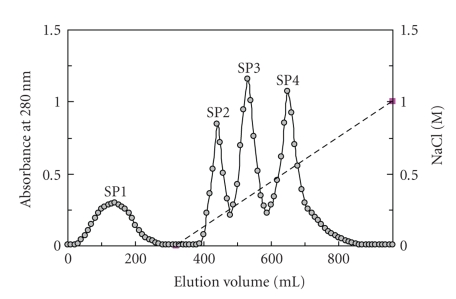
Figure 1.
Results of ion exchange chromatography on SP-Sepharose column (2.5 × 20 cm). The sample is fraction of white cloud bean extract previously adsorbed on DEAE-cellulose and eluted with 0.2 M NaCl added to the buffer, and subsequently unadsorbed on Affi-gel blue gel in the Tris-HCl buffer. Starting buffer for SP-Sepharose chromatography is 10 mM NH4OAc buffer (pH 5). Broken line across the right half of the chromatography represents the linear 0-1 M NaCl gradient in 10 mM NH4OAc buffer (pH 5) employed to elute adsorbed proteins. Trypsin inhibitory activity was found in both fractions SP2 and SP3.