Figure 5.
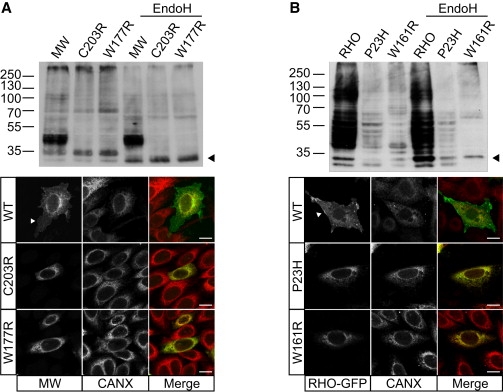
The W177R MW Mutation Causes a Cone Opsin Misfolding Defect Resulting in ER Retention
(A) Immunoblot analysis of SK-N-SH cell lysates (10 ug DM-soluble lysate) transfected with either WT MW opsin (MW), C203R MW mutant or W177R MW mutant, with Endo H treatment as indicated, probed with 1D4 opsin antibody. Both W177R and C203R show fewer glycosylated species as compared to WT and a shift in glycosylated species following Endo H treatment (arrowhead). Expression of WT MW opsin (top), C203R (middle), or W177R (bottom) in SK-N-SH cells detected with 1D4 (green in merged panel) and counterstained for the ER marker calnexin (Cnx, red in merged panel). WT cone opsin (MW) is processed in the ER and targeted to the plasma membrane (arrowhead), reflecting normal biogenesis and traffic of MW opsin, but both mutants were retained in the ER and colocalized with calnexin.
(B) Immunoblot analysis of SK-N-SH cell lysates transfected with WT rod opsin (RHO) and the P23H and W161R RHO mutants, with Endo H treatment as indicated, probed with 1D4 antibody. Endo H treatment results in a shift in glycosylated species of the W161R mutant (arrowhead). Expression of GFP-tagged RHO (top), P23H (middle), and W161R (bottom) (green) counterstained for calnexin (red). RHO-GFP is detected at the plasma membrane, but both mutant proteins are retained in the ER.
