Abstract
Although the timing with which common epithelial malignancies arise and become established remains a matter of debate, it is clear that by the time they are detected these tumors harbor hundreds of deregulated, aberrantly expressed or mutated genes. This enormous complexity poses formidable challenges to identify gene pathways that are drivers of tumorigenesis, potentially suitable for therapeutic intervention. An alternative approach is to consider cancer pathways as interconnected networks, and search for potential nodal proteins capable of connecting multiple signaling networks of tumor maintenance. We have modeled this approach in advanced prostate cancer, a condition with current limited therapeutic options. We propose that the integration of three signaling networks, including chaperone-mediated mitochondrial homeostasis, integrin-dependent cell signaling, and Runx2-regulated gene expression in the metastatic bone microenvironment plays a critical role in prostate cancer maintenance, and offers novel options for molecular therapy.
Keywords: PROSTATE CANCER, SIGNALING, REGULATORY NETWORKS, RUNX, SURVIVIN, INTEGRINS
CANCER PATHWAYS AND CANCER NETWORKS IN TARGETED THERAPY
Cancer treatment now aims at disabling signaling mechanisms essential for tumor maintenance without affecting normal tissues, that is, targeted therapy [Sawyers, 2004; Strausberg et al., 2004]. This is urgently needed because mainstay anticancer agents, such as cytotoxics [Chabner and Roberts, 2005], and radiation [Bernier et al., 2004], have reached a plateau in the management of many cancers, and their efficacy is invariably reduced by side effects, and drug resistance [Stein et al., 2004]. As pioneered by the BCR-ABL kinase inhibitor, Imatinib mesylate [O’Dwyer and Druker, 2000], targeted cancer therapy is feasible and can produce spectacular clinical responses [Deininger et al., 2005]. In addition, tumors can become “addicted” to a primary oncogenic lesion [Weinstein and Joe, 2006], and targeted therapy of these pathways may generate impressive responses, at least in certain patients [Sharma et al., 2007]. Finally, the recent availability of genome-wide profiling of tumors [Perou et al., 2000; van de Vijver et al., 2002], may help tailor targeted intervention for likely responders, and realize the concept of “personalized cancer therapy” [Drews, 2006].
Despite these gains [Sawyers, 2004], the enormous genetic heterogeneity of seemingly identical tumors [Vogelstein and Kinzler, 2004], with hundreds of mutated, amplified or deregulated genes [Sjoblom et al., 2006; Wood et al., 2007], makes it difficult to identify in most cases a single, “driving” signaling pathway suitable for therapeutic intervention. For this reason, traditional, “target-centric’ drug discovery pursuing the development of “Imatinib-like” agents [Guillemard and Saragovi, 2004], has produced less than optimal results [Butcher, 2005]. Costly, labor intensive, and low yield (~1 in a million high throughput hits makes it to the clinic) [van der Greef and McBurney, 2005], this approach has generated many hopeful drugs, which all too often produced modest, or no gains in cancer patients [Schein and Scheffler, 2006].
As an alternative, efforts have begun to exploit systems biology tools [Araujo and Liotta, 2006] to model cancer pathways in their globality, rather than focusing on individual genes [Rajasethupathy et al., 2005]. Connectivity maps [Lamb et al., 2006] linking together multiple signaling mechanisms of tumor maintenance [Lamb, 2007], may more faithfully recapitulate the “tumor tactics” [Kitano, 2003] responsible for treatment failure, including pathway redundancy, buffering, and modularity into semi-autonomous sub-networks [Butcher, 2005; Rajasethupathy et al., 2005]. From a therapeutic standpoint, analysis of cancer networks may identify “nodal” or “hub” proteins [van der Greef and McBurney, 2005], molecules that integrate multiple sub-networks, with essential roles in tumor maintenance [Butcher, 2005; Rajasethupathy et al., 2005]. An example of a cancer nodal protein is the EGF receptor [Citri and Yarden, 2006], which connects extracellular cues to panoply of downstream intracellular responses [Sharma et al., 2007]. For their properties, nodal proteins are prime targets for a novel “pathway-oriented” drug discovery. In this context, antagonists of these molecules may function as global pathway inhibitors [Butcher, 2005; van der Greef and McBurney, 2005], simultaneously disabling multiple signaling networks regardless of tumor heterogeneity.
CHALLENGES OF ADVANCED PROSTATE CANCER
Although significant gains have been made in the management of the early phases of prostate cancer, when expansion and maintenance of the transformed cell population is largely fueled by hormone-dependency, the evolution of prostate cancer to a hormone-independent stage invariably signals advanced disease, with limited therapeutic options and poor prognosis. Although such progression requires decades to become clinically relevant [Draisma et al., 2003], and only in certain cases [Carter, 2006], the acquisition of independence from chemical or surgical castration is often fatal within 24 months [Berthold et al., 2008]. At a molecular level, this involves a poorly understood cascade of events, but clearly reflecting enormous molecular, cellular and genetic heterogeneity, including amplification of the androgen receptor locus with hypersensitivity at low hormone concentrations [Chen et al., 2004], promiscuous receptor activation by non hormone-regulated molecules, including growth factor receptors [Culig et al., 1994], or cytokines [Wallner et al., 2006], and clonal selection of androgen-independent tumor cells [Collins et al., 2005]. Advanced prostate cancer is also associated with metastatic dissemination, typically to the bones, causing both osteoblastic and osteolytic lesions [Loberg et al., 2005]. The therapeutic options for these patients are limited, and only docetaxel-based chemotherapy, together with biphosphonate palliation of bone lesions, has been shown to modestly prolong survival.
With the realization of the extreme complexity of advanced prostate cancer, several new therapeutic strategies are being envisioned to disable multiple networks of tumor maintenance, rather than an individual signaling pathway. These include growth factor receptor signaling, angiogenesis, the “tumor microenvironment,” various anti-apoptotic mechanisms, integrin-mediated cell adhesion, as well as enhancing antitumoral immunity [reviewed in Taichman et al., 2007]. Hsp90 inhibition is also being considered in this setting, with the hope of disabling signaling kinases and non-hormone regulated androgen receptor activation [Taichman et al., 2007]. Although promising, it is too soon to tell whether any of these “pathway-oriented” approaches will have a meaningful impact in the clinic. At the present time, advanced and metastatic prostate cancer remains a deadly disease, with only palliative therapeutic options, and an area in urgent need of new molecular and translational research advances. In this context, recent collaborative work has identified three interconnected signaling networks of pivotal significance in the pathogenesis and progression of advanced prostate cancer. These include a novel pathway of mitochondrial homeostasis regulated by Hsp90 molecular chaperones, a pleiotropic signaling cascade initiated by the integrins at the cell surface, and a transcriptional network orchestrated in the bone microenvironment by Runx2. Each of these interconnected networks is regulated by unique nodal proteins, which provide unique therapeutic opportunities for “pathway-oriented” drug discovery.
THE FIRST PROSTATE CANCER REGULATORY SUBNETWORK: HSP90 CHAPERONE CONTROL OF MITOCHONDRIAL HOMEOSTASIS
Mitochondrial dysfunction plays a pivotal role in the initiation of apoptosis, or programmed cell death [Green and Kroemer, 2004]. Triggered by disparate stimuli, this process involves a complex molecular cascade [Ferri and Kroemer, 2001], characterized by increased permeability of the mitochondrial inner membrane, loss of membrane potential, swelling of the matrix, and rupture of the outer membrane [Kroemer and Reed, 2000; Green and Kroemer, 2004]. In turn, damaged mitochondria release apoptogenic proteins, in particular cytochrome c in the cytosol [Zamzami and Kroemer, 2001], which mediates activation of initiator and effector caspases [Hengartner, 2000]. How this “mitochondrial permeability transition” is regulated in not completely understood, but what it is clear is that mechanisms to antagonize its execution are often exploited or subverted in tumor cells. Pro-apoptotic Bcl-2 molecules [Cory and Adams, 2002], including multi-domain Bax and Bak [Wei et al., 2001], or so-called “BH3-only” members, contribute to permeabilize the outer membrane, with release of cytochrome c [Green and Kroemer, 2004]. Conversely, the molecular organization of a mitochondrial permeability transition “pore” [Crompton et al., 1999], which mediates swelling of the matrix and depolarization of the inner membrane, has remained elusive. Based on knockout studies in mice, two long-held constituents of this pore, the voltage-dependent anion channel (VDAC) [Baines et al., 2007], and the adenine nucleotide translocator (ANT) [Kokoszka et al., 2004], turned out to be dispensable for cell death. Instead, knockout data showed that the matrix peptidyl prolyl-cis, trans isomerase immunophilin, Cyclophilin D (CypD) [Woodfield et al., 1998], was indispensable for mitochondrial permeability transition, especially in response to oxidative stress or Ca2+ overload [Baines et al., 2005; Nakagawa et al., 2005; Schinzel et al., 2005].
How CypD function is regulated is not completely clear, but this process may involve protein folding mechanisms. Accordingly, it has been proposed that assembly of a permeability transition pore may be a dynamic process, in which mitochondrial damage, such as Ca2+ overload or reactive oxygen species, generates clusters of unfolded proteins that ultimately promote opening of a CypD-containing pore [He and Lemasters, 2002]. This model predicts that protein refolding mechanisms in mitochondria (see below) may be ideally suited to counterbalance permeability transition, prevent CypD-mediated pore opening, and preserve organelle integrity [He and Lemasters, 2003]. Other regulators of mitochondrial cytoprotection have also been described, including a pool of the Inhibitor of Apoptosis (IAP) protein [Eckelman et al., 2006], survivin [Altieri, 2008]. Mitochondrial survivin may oppose the release of apoptogenic proteins, cooperatively inhibit caspase activation in the cytosol [Dohi et al., 2004, 2007], or intrinsically regulate the permeability transition pore in mitochondria. Despite these gaps in our understanding of mitochondrial homeostasis, efforts to manipulate these pathways and trigger apoptosis in cancer cells [Fesik, 2005; Oltersdorf et al., 2005], have recently reached the clinic [Johnstone et al., 2002]. However, it is unclear whether these approaches can selectively discriminate between normal and transformed cells [Verma et al., 2003; Foster et al., 2006], or whether the extreme redundancy of Bcl-2 proteins as regulators of outer mitochondrial integrity may ultimately result in emergence of drug resistance [Konopleva et al., 2006; Deng et al., 2007].
Recent studies identified an abundant pool of Hsp90, and its related chaperone, TRAP-1 [Felts et al., 2000], in mitochondria of tumor, but not most normal tissues, in vivo [Kang et al., 2007]. Expression of TRAP-1 is particularly abundant in advanced prostate cancer with high Gleason scores, and prostate cancer metastasis to bones and lymph nodes, but undetectable in normal prostate, or prostatic intraepithelial neoplasia, in vivo. Although the basis for this “tumor-specific” localization is unclear, mitochondrial Hsp90 chaperones function as novel CypD-associated molecules, in a recognition that requires the isomerase activity of CypD [Kang et al., 2007]. In turn, this interaction antagonizes CypD-mediated pore-forming function, prevents permeability transition, and suppresses the initiation of apoptosis [Kang et al., 2007]. Cytoprotection by mitochondrial Hsp90 requires the chaperone protein folding activity [He and Lemasters, 2002], and is essential to maintain organelle integrity. Accordingly, a peptidomimetic Hsp90 inhibitor [Meli et al., 2006], Shepherdin [Plescia et al., 2005], capable to accumulate in mitochondria induced collapse of organelle homeostasis, with loss of membrane potential, release of cytochrome c, and massive apoptosis [Kang et al., 2007]. In contrast, normal cell types that do not have Hsp90 in mitochondria were not affected [Kang et al., 2007], including CD34+ hematopoietic progenitor cells [Plescia et al., 2005; Gyurkocza et al., 2006]. Recent studies independently confirmed a general cytoprotective function of mitochondrial Hsp90 chaperones, including TRAP-1, and established their role in inhibition of cytochrome c release [Masuda et al., 2004], and suppression of apoptosis [Hua et al., 2007], especially in response to oxidative stress [Pridgeon et al., 2007].
THE SECOND PROSTATE CANCER REGULATORY SUBNETWORK: SIGNALING BY αV INTEGRINS
Integrins comprise a family of cell surface receptors composed of non-covalently bound α and β subunits, which can combine in at least 24 different complexes [Alam et al., 2007]. These molecules mediate attachment of cells to the extracellular matrix (ECM) and have also been implicated in activation of disparate signaling pathways [Hynes, 2002; Alam et al., 2007]. In cancer, integrin signaling is exploited to affect cellular growth and tumor progression by controlling apoptosis, cell adhesion, proliferation, gene expression, and migration [Felding-Habermann, 2003; Akalu et al., 2005]. In addition, integrin signaling has been shown to act as a mechanism to regulate proteinase expression [Munshi and Stack, 2006]. These mechanisms are particularly relevant in prostate cancer, where tumor cells have a different surrounding matrix compared to normal cells, so that changes in integrin profile may functionally contribute to the growth and establishment of primary and metastatic foci [Fornaro et al., 2001; Demetriou and Cress, 2004; Goel et al., 2008]. Several studies have associated deregulated integrin expression with the progression of prostate cancer to an advanced stage [Knox et al., 1994; Murant et al., 1997; Goel et al., 2008]. In this context, most α and β subunits have been shown to be downregulated in prostate cancer, whereas predominantly α6 and αV integrins are upregulated [Goel et al., 2008], suggesting a potential role for these receptors in the progression of this disease toward an androgen-independent castration-resistant metastatic state. Although the molecular pathways by which integrins contribute to cancer progression and metastasis need to be fully elucidated, designing new therapeutic approaches for prostate cancer based on inhibiting integrin functions, integrin cleavage or integrin downstream signaling is likely to be a successful strategy.
Many efforts have been made, to inhibit prostate cancer metastasis to bone, the most common metastatic site for this disease; however, the current therapies are not very efficacious. Since integrins mediate the interactions between tumor cells and the bone microenvironment, a potential application of the use of integrin inhibitors is to prevent prostate cancer growth in bone [Waltregny et al., 2000; Pecheur et al., 2002; Karadag et al., 2004; Hall et al., 2006; King et al., 2008]. A recent study has shown that the αvβ3 integrin promotes bone gain mediated by metastatic prostate cancer cells and suggest that αvβ3 is a potential therapeutic target to block prostate cancer osteoblastic lesions [Keller and Brown, 2004; McCabe et al., 2007]. In this context, evidence has been provided supporting a role for αv integrins in prostate cancer cell survival in bone [Bisanz et al., 2005].
In conclusion, these promising investigations indicate that the clinical use of integrins’ inhibitors spans all stages of cancer progression from inhibition of tumor growth to inhibition of metastasis.
THE THIRD PROSTATE CANCER REGULATORY SUBNETWORK: RUNX2 CONTROL OF GENE EXPRESSION IN THE BONE METASTATIC MICROENVIRONMENT
As indicated above, one the most common and, unfortunately, most severe developments in prostate cancer progression is the emergence of metastatic lesions to the bone [Cereceda et al., 2003]. Patients with bone metastases have severe bone pain, spinal cord compression, and osteolysis, which compromises structural integrity of bone with increased susceptibility to fractures [Roodman, 2004]. Prostate cancers that metastasize to bone secrete factors (e.g., endothelin-1, BMP2) that result primarily in osteoblastic lesions, as well as osteolytic bone disease [Keller and Brown, 2004; Roudier et al., 2008] induced by secreted PTHrP and TGFβ [Bendre et al., 2003; Kingsley et al., 2007; Pratap et al., 2008]. It is now appreciated from animal models that osteolysis occurs prior to the osteoblastic lesions in prostate cancer metastatic bone disease.
Considerable effort has been devoted to map the requirements of bone lesions in prostate tumors. Experimental evidence suggests that osteoblast lesions originate from the recruitment of bone-forming cells into the tumor environment [Li et al., 2008b], and this process is also contributed by the expression of transcription factors by prostate cancer cells activating bank of genes with osteomimetic properties, potentially contributing to formation of woven bone within the tumor [Guise et al., 2006]. Thus, the metastasis of prostate cells to bone is a continuum of degeneration of the skeleton with ectopic bone formation in the tumor, often associated with resistance to conventional therapy [FitzGerald et al., 2008]. In the past few years, bioinformatics approaches combined with micro-array gene profiling of primary tumors and cell lines have provided important data for identification of gene signatures of disease progression [Dairkee et al., 2004; Smid et al., 2006]. In this context, recent data have demonstrated that Runx2, a transcription factor essential for osteogenesis, becomes highly activated in prostate cancer cells that metastasize to bone, and is detected in human and mouse prostate cancer tissue, but not normal prostate, in vivo [Yang et al., 2004].
Recent studies have expanded this view, and identified Runx2 as a key regulator of bone metastasis [Pratap et al., 2006]. When abnormally expressed in tumor cells, Runx2 has pathological functions that are deregulated compared to normal cells: Runx2 is no longer antiproliferative, and instead appears to have oncogenic properties, as demonstrated by synergism with c-Myc [Vaillant et al., 1999; Blyth et al., 2001], and in promoting aggressive tumor growth in the bone [Barnes et al., 2004]. At a molecular level, Runx2-mediated tumor progression and metastasis involves regulated interactions with co-regulatory molecules, including chromatin remodeling factors, intracellular mediators of signaling pathways and other transcription factors [Lian et al., 2004; Pratap et al., 2006]. In prostate cancer [Brubaker et al., 2004], Runx2 has been associated with the osteomimetic properties of bone metastatic cells [Zayzafoon et al., 2004; Pratap et al., 2006], via transcription of genes implicated in osteoblastic lesions [Zhang et al., 2003; Brubaker et al., 2004; Dai et al., 2004]. These include ECM proteins (osteocalcin, bone sialoprotein, and osteopontin), signaling molecules (vascular endothelial growth factor), and enzymes involved in bone turnover (matrix metalloproteinases) [Yang et al., 2001; Pratap et al., 2006]. In contrast, non-metastatic cells exhibit low levels of Runx2 [Brubaker et al., 2003; Inman and Shore, 2003; Barnes et al., 2004; Selvamurugan et al., 2004; Javed et al., 2005; Pratap et al., 2005].
A UNIFIED AND INTEGRATED PROSTATE CANCER REGULATORY NETWORK: IMPLICATIONS FOR DISEASE PROGRESSION AND PATHWAY-ORIENTED DRUG DISCOVERY
Recent experimental evidence suggests that the three regulatory networks outlined above are extensively interconnected, sharing common signaling pathways, and utilizing a common set of effector and nodal molecules. In addition, because of their synergistic role in fundamental mechanisms of disease progression and metastatic dissemination, these pathways and their associated nodal proteins may provide novel opportunities for pathway-oriented drug discovery. Specifically, analysis of subnetwork interactions using systems biology tools reveals an extensive degree of connectivity (Fig. 1). The first Hsp90 subnetwork interfaces extensively with Runx2 regulation of gene transcription in the bone microenvironment, controls multiple pathways of cell survival often exploited in prostate cancer, and regulates the stability and function of multiple effector molecules of integrin signaling (Fig. 1). The second subnetwork of αV integrin-initiated signal transduction also interfaces with critical components of mitochondrial cell death, preserving cell viability, controlling Runx2 transcriptional activity through modifications in Runx2 phosphorylation [Sun et al., 2001; Chang et al., 2008], and integrates matrix metalloproteinase and TGFβ signals of pivotal importance for metastatic dissemination, especially to the bones (Fig. 1). This is mirrored by a comparable set of interactions involving the third subnetwork of Runx2-dependent gene expression, which affects integrin expression and signaling, mitochondrial integrity via Bax regulation of outer membrane permeability, and modulation of TGFβ responses in both early and late events of prostate cancer tumorigenesis, and metastatic bone disease [Mundy, 2002; Buijs et al., 2007; Nguyen and Massague, 2007; Baselga et al., 2008; Pratap et al., 2008; Li et al., 2008a] (Fig. 1). In addition, this integrated regulatory network utilizes common nodal proteins. Survivin is a regulator of apoptosis participating in prostate cancer progression [Altieri, 2008] that is implicated in mitochondrial homeostasis, and whose expression in prostate cancer is controlled by both Runx2- and integrin-initiated signaling. Similarly, Hsp90 homeostasis has also been implicated in preservation of mitochondrial integrity [Kang et al., 2007], but also in the control of pivotal client proteins [Whitesell and Lindquist, 2005] of the second and third subnetworks, including TGFβ, and androgen receptor (AR), as well as in the direct contribution of cell invasion and metastasis [Eustace et al., 2004].
Fig. 1.
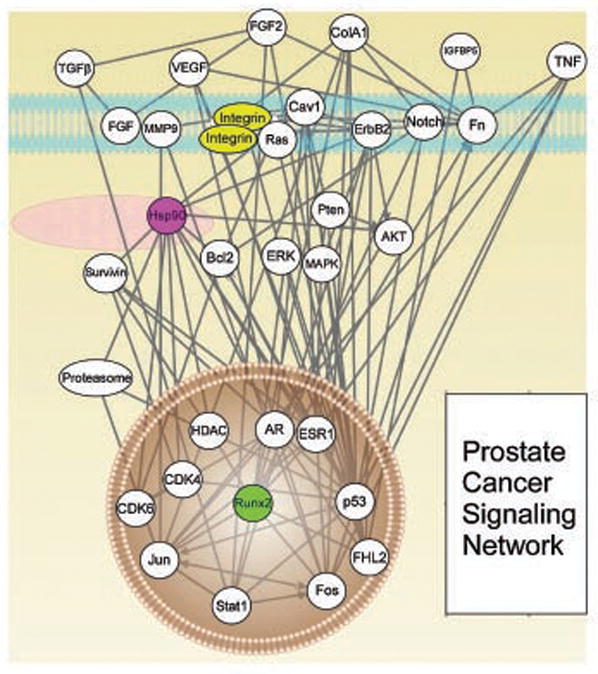
Prostate Cancer Signaling Network. The integration of regulatory pathways in plasma membrane (integrins), cytosol (Hsp90), and nucleus (Runx2) that provide therapeutic targets in prostate cancer is indicated.
In this context, it may be possible to envision the development and characterization of a novel set of “network inhibitors” capable of targeting the nodal proteins in this integrated set of pathways. Although small molecule antagonists of Hsp90 have now reached the clinic, their therapeutic efficacy as single agents has been modest, at best, generally below the expectations for these agents to function as genuine pathway antagonists. The regulatory network outlined above suggests that the segregation of Hsp90 in specialized subcellular compartments, including mitochondria, may provide novel options for the development of targeted inhibitors. In this context, proof-of-principle experiments to target Hsp90 inhibitors to mitochondria have produced encouraging results, causing mitochondrial collapse in tumor cells, accompanied by sudden and massive cell death and inhibition of tumor growth in preclinical experiments, in vivo. Similar considerations apply to the potential role of integrins as cell surface receptors, drugable targets. In this context, the αV integrins are emerging as an attractive molecular target for inhibition of an integrated network of cell invasion and migration, including pleiotropic TGFβ signaling responses. This may be particularly relevant in prostate cancer, where interference with metastatic bone colonization frequently involves deregulation of TGFβ functions. Lastly, although transcription factors are typically considered non-drugable, therapeutic targeting of Runx2 by local delivery of short hairpin RNA (shRNA) could interrupt an integrated network of gene expression required to maintain the metastatic niche in the bone microenvironment, and concomitantly deregulate cell survival and cell migration pathways of invading prostate cancer cells.
Acknowledgments
This study was supported by the grant NIH (CA788180, HL54131, CA90917 to D.C.A., CA89720, CA109874 to L.R.L., AR48818, CA82834 to G.S.S.).
Grant sponsor: NIH; Grant numbers: CA788180, HL54131, CA90917, CA89720, CA109874, CA82834, AR48818.
Footnotes
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- Akalu A, Cretu A, Brooks PC. Targeting integrins for the control of tumour angiogenesis. Expert Opin Investig Drugs. 2005;14:1475–1486. doi: 10.1517/13543784.14.12.1475. [DOI] [PubMed] [Google Scholar]
- Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- Araujo RP, Liotta LA. A control theoretic paradigm for cell signaling networks: A simple complexity for a sensitive robustness. Curr Opin Chem Biol. 2006;10:81–87. doi: 10.1016/j.cbpa.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GL, Hebert KE, Kamal M, Javed A, Einhorn TA, Lian JB, Stein GS, Gerstenfeld LC. Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases associated osteolytic disease. Cancer Res. 2004;64:4506–4513. doi: 10.1158/0008-5472.CAN-03-3851. [DOI] [PubMed] [Google Scholar]
- Baselga J, Rothenberg ML, Tabernero J, Seoane J, Daly T, Cleverly A, Berry B, Rhoades SK, Ray CA, Fill J, Farrington DL, Wallace LA, Yingling JM, Lahn M, Arteaga C, Carducci M. TGF-beta signalling-related markers in cancer patients with bone metastasis. Biomarkers. 2008;13:217–236. doi: 10.1080/13547500701676019. [DOI] [PubMed] [Google Scholar]
- Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- Bernier J, Hall EJ, Giaccia A. Radiation oncology: A century of achievements. Nat Rev Cancer. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- Bisanz K, Yu J, Edlund M, Spohn B, Hung MC, Chung LW, Hsieh CL. Targeting ECM-integrin interaction with liposome-encapsulated small interfering RNAs inhibits the growth of human prostate cancer in a bone xenograft imaging model. Mol Ther. 2005;12:634–643. doi: 10.1016/j.ymthe.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Blyth K, Terry A, Mackay N, Vaillant F, Bell M, Cameron ER, Neil JC, Stewart M. Runx2: A novel oncogenic effector revealed by in vivo complementation and retroviral tagging. Oncogene. 2001;20:295–302. doi: 10.1038/sj.onc.1204090. [DOI] [PubMed] [Google Scholar]
- Brubaker KD, Vessella RL, Brown LG, Corey E. Prostate cancer expression of runt-domain transcription factor Runx2, a key regulator of osteoblast differentiation and function. Prostate. 2003;56:13–22. doi: 10.1002/pros.10233. [DOI] [PubMed] [Google Scholar]
- Brubaker KD, Corey E, Brown LG, Vessella RL. Bone morphogenetic protein signaling in prostate cancer cell lines. J Cell Biochem. 2004;91:151–160. doi: 10.1002/jcb.10679. [DOI] [PubMed] [Google Scholar]
- Buijs JT, Henriquez NV, van Overveld PG, van der HG, ten Dijke P, van der PG. TGF-beta and BMP7 interactions in tumour progression and bone metastasis. Clin Exp Metastasis. 2007;24:609–617. doi: 10.1007/s10585-007-9118-2. [DOI] [PubMed] [Google Scholar]
- Butcher EC. Can cell systems biology rescue drug discovery? Nat Rev Drug Discov. 2005;4:461–467. doi: 10.1038/nrd1754. [DOI] [PubMed] [Google Scholar]
- Carter HB. Assessing risk: Does this patient have prostate cancer? J Natl Cancer Inst. 2006;98:506–507. doi: 10.1093/jnci/djj155. [DOI] [PubMed] [Google Scholar]
- Cereceda LE, Flechon A, Droz JP. Management of vertebral metastases in prostate cancer: A retrospective analysis in 119 patients. Clin Prostate Cancer. 2003;2:34–40. doi: 10.3816/cgc.2003.n.010. [DOI] [PubMed] [Google Scholar]
- Chabner BA, Roberts TG., Jr Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- Chang SF, Chang CA, Lee DY, Lee PL, Yeh YM, Yeh CR, Cheng CK, Chien S, Chiu JJ. Tumor cell cycle arrest induced by shear stress: Roles of integrins and Smad. Proc Natl Acad Sci USA. 2008;105:3927–3932. doi: 10.1073/pnas.0712353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Crompton M, Virji S, Doyle V, Johnson N, Ward JM. The mitochondrial permeability transition pore. Biochem Soc Symp. 1999;66:167–179. doi: 10.1042/bss0660167. [DOI] [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- Dai J, Kitagawa Y, Zhang J, Yao Z, Mizokami A, Cheng S, Nor J, McCauley LK, Taichman RS, Keller ET. Vascular endothelial growth factor contributes to the prostate cancer-induced osteoblast differentiation mediated by bone morphogenetic protein. Cancer Res. 2004;64:994–999. doi: 10.1158/0008-5472.can-03-1382. [DOI] [PubMed] [Google Scholar]
- Dairkee SH, Ji Y, Ben Y, Moore DH, Meng Z, Jeffrey SS. A molecular ‘signature’ of primary breast cancer cultures; patterns resembling tumor tissue. BMC Genomics. 2004;5:47. doi: 10.1186/1471-2164-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- Demetriou MC, Cress AE. Integrin clipping: A novel adhesion switch? J Cell Biochem. 2004;91:26–35. doi: 10.1002/jcb.10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal CP, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi T, Xia F, Altieri DC. Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection. Mol Cell. 2007;27:17–28. doi: 10.1016/j.molcel.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RA, Schroder FH, de Koning HJ. Lead times and overdetection due to prostate-specific antigen screening: Estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868–878. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- Drews J. Case histories, magic bullets and the state of drug discovery. Nat Rev Drug Discov. 2006;5:635–640. doi: 10.1038/nrd2084. [DOI] [PubMed] [Google Scholar]
- Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: Why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW, Beste G, Scroggins BT, Neckers L, Ilag LL, Jay DG. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275:3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- FitzGerald TJ, Wang T, Goel HL, Huang J, Stein G, Lian J, Davis RJ, Doxsey S, Balaji KC, Aronowitz J, Languino LR. Prostate carcinoma and radiation therapy: Therapeutic treatment resistance and strategies for targeted therapeutic intervention. Expert Rev Anticancer Ther. 2008;8:967–974. doi: 10.1586/14737140.8.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20:321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- Foster LJ, de Hoog CL, Zhang Y, Zhang Y, Xie X, Mootha VK, Mann M. A mammalian organelle map by protein correlation profiling. Cell. 2006;125:187–199. doi: 10.1016/j.cell.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15:657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Guillemard V, Saragovi HU. Novel approaches for targeted cancer therapy. Curr Cancer Drug Targets. 2004;4:313–326. doi: 10.2174/1568009043332989. [DOI] [PubMed] [Google Scholar]
- Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- Gyurkocza B, Plescia J, Raskett CM, Garlick DS, Lowry PA, Carter BZ, Andreeff M, Meli M, Colombo G, Altieri DC. Antileukemic activity of shepherdin and molecular diversity of hsp90 inhibitors. J Natl Cancer Inst. 2006;98:1068–1077. doi: 10.1093/jnci/djj300. [DOI] [PubMed] [Google Scholar]
- Hall CL, Dai J, van Golen KL, Keller ET, Long MW. Type I collagen receptor (alpha 2 beta 1) signaling promotes the growth of human prostate cancer cells within the bone. Cancer Res. 2006;66:8648–8654. doi: 10.1158/0008-5472.CAN-06-1544. [DOI] [PubMed] [Google Scholar]
- He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: A new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- He L, Lemasters JJ. Heat shock suppresses the permeability transition in rat liver mitochondria. J Biol Chem. 2003;278:16755–16760. doi: 10.1074/jbc.M300153200. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hua G, Zhang Q, Fan Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J Biol Chem. 2007;282:20553–20560. doi: 10.1074/jbc.M703196200. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Inman CK, Shore P. The osteoblast transcription factor Runx2 is expressed in mammary epithelial cells and mediates osteopontin expression. J Biol Chem. 2003;278:48684–48689. doi: 10.1074/jbc.M308001200. [DOI] [PubMed] [Google Scholar]
- Javed A, Barnes GL, Pratap J, Antkowiak T, Gerstenfeld LC, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Impaired intranuclear trafficking of Runx2 (AML3/CBFA1) transcription factors in breast cancer cells inhibits osteolysis in vivo. Proc Natl Acad Sci USA. 2005;102:1454–1459. doi: 10.1073/pnas.0409121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: A link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Karadag A, Ogbureke KU, Fedarko NS, Fisher LW. Bone sialoprotein, matrix metalloproteinase 2, and alpha(v)beta3 integrin in osteotropic cancer cell invasion. J Natl Cancer Inst. 2004;96:956–965. doi: 10.1093/jnci/djh169. [DOI] [PubMed] [Google Scholar]
- Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91:718–729. doi: 10.1002/jcb.10662. [DOI] [PubMed] [Google Scholar]
- King TE, Pawar SC, Majuta L, Sroka IC, Wynn D, Demetriou MC, Nagle RB, Porreca F, Cress AE. The role of alpha 6 integrin in prostate cancer migration and bone pain in a novel xenograft model. PLoS ONE. 2008;3:e3535. doi: 10.1371/journal.pone.0003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- Kitano H. Cancer robustness: Tumour tactics. Nature. 2003;426:125. doi: 10.1038/426125a. [DOI] [PubMed] [Google Scholar]
- Knox JD, Cress AE, Clark V, Manriquez L, Affinito KS, Dalkin BL, Nagle RB. Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. Am J Pathol. 1994;145:167–174. [PMC free article] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Lamb J. The Connectivity Map: A new tool for biomedical research. Nat Rev Cancer. 2007;7:54–60. doi: 10.1038/nrc2044. [DOI] [PubMed] [Google Scholar]
- Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, Reich M, Hieronymus H, Wei G, Armstrong SA, Haggarty SJ, Clemons PA, Wei R, Carr SA, Lander ES, Golub TR. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- Li X, Placencio V, Iturregui JM, Uwamariya C, Sharif-Afshar AR, Koyama T, Hayward SW, Bhowmick NA. Prostate tumor progression is mediated by a paracrine TGF-beta/Wnt3a signaling axis. Oncogene. 2008a;27:7118–7130. doi: 10.1038/onc.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZG, Yang J, Vazquez ES, Rose D, Vakar-Lopez F, Mathew P, Lopez A, Logothetis CJ, Lin SH, Navone NM. Low-density lipoprotein receptor-related protein 5 (LRP5) mediates the prostate cancer-induced formation of new bone. Oncogene. 2008b;27:596–603. doi: 10.1038/sj.onc.1210694. [DOI] [PubMed] [Google Scholar]
- Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. Regulatory controls for osteoblast growth and differentiation: Role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- Loberg RD, Logothetis CJ, Keller ET, Pienta KJ. Pathogenesis and treatment of prostate cancer bone metastases: Targeting the lethal phenotype. J Clin Oncol. 2005;23:8232–8241. doi: 10.1200/JCO.2005.03.0841. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Shima G, Aiuchi T, Horie M, Hori K, Nakajo S, Kajimoto S, Shibayama-Imazu T, Nakaya K. Involvement of tumor necrosis factor receptor-associated protein 1 (TRAP1) in apoptosis induced by beta-hydroxyisovalerylshikonin. J Biol Chem. 2004;279:42503–42515. doi: 10.1074/jbc.M404256200. [DOI] [PubMed] [Google Scholar]
- McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–6243. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli M, Pennati M, Curto M, Daidone MG, Plescia J, Toba S, Altieri DC, Zaffaroni N, Colombo G. Small-molecule targeting of heat shock protein 90 chaperone function: Rational identification of a new anticancer lead. J Med Chem. 2006;49:7721–7730. doi: 10.1021/jm060836y. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- Munshi HG, Stack MS. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006;25:45–56. doi: 10.1007/s10555-006-7888-7. [DOI] [PubMed] [Google Scholar]
- Murant SJ, Handley J, Stower M, Reid N, Cussenot O, Maitland NJ. Co-ordinated changes in expression of cell adhesion molecules in prostate cancer. Eur J Cancer. 1997;33:263–271. doi: 10.1016/s0959-8049(96)00418-2. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- O’Dwyer ME, Druker BJ. ST I571: An inhibitor of the BCR-ABL tyrosine kinase for the treatment of chronic myelogenous leukaemia. Lancet Oncol. 2000;1:207–211. doi: 10.1016/s1470-2045(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Pecheur I, Peyruchaud O, Serre CM, Guglielmi J, Voland C, Bourre F, Margue C, Cohen-Solal M, Buffet A, Kieffer N, Clezardin P. Integrin alpha(v)-beta3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 2002;16:1266–1268. doi: 10.1096/fj.01-0911fje. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Plescia J, Salz W, Xia F, Pennati M, Zaffaroni N, Daidone MG, Meli M, Dohi T, Fortugno P, Nefedova Y, Gabrilovich DI, Colombo G, Altieri DC. Rational design of shepherdin, a novel anticancer agent. Cancer Cell. 2005;7:457–468. doi: 10.1016/j.ccr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Pratap J, Javed A, Languino LR, van Wijnen AJ, Stein JL, Stein GS, Lian JB. The Runx2 osteogenic transcription factor regulates matrix metallo-proteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol. 2005;25:8581–8591. doi: 10.1128/MCB.25.19.8581-8591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratap J, Lian JB, Javed A, Barnes GL, van Wijnen AJ, Stein JL, Stein GS. Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev. 2006;25:589–600. doi: 10.1007/s10555-006-9032-0. [DOI] [PubMed] [Google Scholar]
- Pratap J, Wixted JJ, Gaur T, Zaidi SK, Dobson J, Gokul KD, Hussain S, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Runx2 transcriptional activation of Indian hedgehog and a downstream bone metastatic pathway in breast cancer cells. Cancer Res. 2008;68:7795–7802. doi: 10.1158/0008-5472.CAN-08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, Vayttaden SJ, Bhalla US. Systems modeling: A pathway to drug discovery. Curr Opin Chem Biol. 2005;9:400–406. doi: 10.1016/j.cbpa.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- Roudier MP, Morrissey C, True LD, Higano CS, Vessella RL, Ott SM. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol. 2008;180:1154–1160. doi: 10.1016/j.juro.2008.04.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers C. Targeted cancer therapy. Nature. 2004;432:294–297. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- Schein PS, Scheffler B. Barriers to efficient development of cancer therapeutics. Clin Cancer Res. 2006;12:3243–3248. doi: 10.1158/1078-0432.CCR-06-0329. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamurugan N, Kwok S, Partridge NC. Smad3 interacts with JunB and Cbfa1/Runx2 for transforming growth factor-beta1-stimulated collagenase-3 expression in human breast cancer cells. J Biol Chem. 2004;279:27764–27773. doi: 10.1074/jbc.M312870200. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- Smid M, Wang Y, Klijn JG, Sieuwerts AM, Zhang Y, Atkins D, Martens JW, Foekens JA. Genes associated with breast cancer metastatic to bone. J Clin Oncol. 2006;24:2261–2267. doi: 10.1200/JCO.2005.03.8802. [DOI] [PubMed] [Google Scholar]
- Stein WD, Bates SE, Fojo T. Intractable cancers: The many faces of multidrug resistance and the many targets it presents for therapeutic attack. Curr Drug Targets. 2004;5:333–346. doi: 10.2174/1389450043345489. [DOI] [PubMed] [Google Scholar]
- Strausberg RL, Simpson AJ, Old LJ, Riggins GJ. Oncogenomics and the development of new cancer therapies. Nature. 2004;429:469–474. doi: 10.1038/nature02627. [DOI] [PubMed] [Google Scholar]
- Sun L, Vitolo M, Passaniti A. Runt-related gene 2 in endothelial cells: Inducible expression and specific regulation of cell migration and invasion. Cancer Res. 2001;61:4994–5001. [PubMed] [Google Scholar]
- Taichman RS, Loberg RD, Mehra R, Pienta KJ. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007;117:2351–2361. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant F, Blyth K, Terry A, Bell M, Cameron ER, Neil J, Stewart M. A full-length Cbfa1 gene product perturbs T-cell development and promotes lymphomagenesis in synergy with MYC. Oncogene. 1999;18:7124–7134. doi: 10.1038/sj.onc.1203202. [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- van der Greef J, McBurney RN. Innovation: Rescuing drug discovery: In vivo systems pathology and systems pharmacology. Nat Rev Drug Discov. 2005;4:961–967. doi: 10.1038/nrd1904. [DOI] [PubMed] [Google Scholar]
- Verma M, Kagan J, Sidransky D, Srivastava S. Proteomic analysis of cancer-cell mitochondria. Nat Rev Cancer. 2003;3:789–795. doi: 10.1038/nrc1192. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Wallner L, Dai J, Escara-Wilke J, Zhang J, Yao Z, Lu Y, Trikha M, Nemeth JA, Zaki MH, Keller ET. Inhibition of interleukin-6 with CNTO328, an anti-interleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent prostate cancer to an androgen-independent phenotype in orchiectomized mice. Cancer Res. 2006;66:3087–3095. doi: 10.1158/0008-5472.CAN-05-3447. [DOI] [PubMed] [Google Scholar]
- Waltregny D, Bellahcene A, de LX, Florkin B, Weidle U, Castronovo V. Increased expression of bone sialoprotein in bone metastases compared with visceral metastases in human breast and prostate cancers. J Bone Miner Res. 2000;15:834–843. doi: 10.1359/jbmr.2000.15.5.834. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein IB, Joe AK. Mechanisms of disease: Oncogene addiction—A rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol. 2006;3:448–457. doi: 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J. 1998;336(Pt 2):287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Fizazi K, Peleg S, Sikes CR, Raymond AK, Jamal N, Hu M, Olive M, Martinez LA, Wood CG, Logothetis CJ, Karsenty G, Navone NM. Prostate cancer cells induce osteoblast differentiation through a Cbfa1-dependent pathway. Cancer Res. 2001;61:5652–5659. [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Kroemer G. The mitochondrion in apoptosis: How Pandora’s box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- Zayzafoon M, Abdulkadir SA, McDonald JM. Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J Biol Chem. 2004;279:3662–3670. doi: 10.1074/jbc.M308158200. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Tang J, Wang J, Ma W, Zheng W, Yoneda T, Chen J. Over-expression of bone sialoprotein enhances bone metastasis of human breast cancer cells in a mouse model. Int J Oncol. 2003;23:1043–1048. [PubMed] [Google Scholar]


