Abstract
In animal cells growth factors coordinate cell proliferation and survival by regulating the PI3K/Akt signaling pathway. Deregulation of this signaling pathway is common in a variety of human cancers. The PI3K dependent signaling kinase complex defined as mTORC2 functions as a regulatory Ser-473 kinase of Akt. We find that activation of mTORC2 by growth factor signaling is linked to the specific phosphorylation of its component rictor on Thr-1135. The phosphorylation of this site is induced by the growth factor stimulation and expression of the oncogenic forms of ras or PI3K. Rictor phosphorylation is sensitive to inhibition of PI3K, mTOR, or expression of ILK. The substitution of wild-type rictor with its specific phospho-mutants in rictor null mouse embryonic fibroblasts did not alter the growth factor-dependent phosphorylation of Akt indicating that the rictor Thr-1135 phosphorylation is not critical in regulation of the mTORC2 kinase activity. We found that this rictor phosphorylation takes place in the mTORC2-deficient cells suggesting that this modification might play a role in regulation not only mTORC2 but also the mTORC2-independent function of rictor.
Introduction
Growth factor signaling plays a decisive role in the regulation of cell proliferation, survival, and differentiation. Deregulation of growth factor signaling pathways is associated with tumorigenesis and is common in human cancers. Binding of growth factors to their specific tyrosine kinase receptors initiates signaling by activating the kinase activity of receptors (1, 2). It results in the autophosphorylation of the receptor cytoplasmic domains and tyrosine phosphorylation of docking proteins. These tyrosine phosphorylated sites function as a recruitment sites of a wide spectrum of regulatory proteins. Regulated protein/protein interactions are mediated by tyrosine phosphorylation binding Src Homology 2 (SH2) domains and also by proline rich binding SH3 domains (3). Downstream of growth factor receptor crucial signaling controlling cell proliferation and survival is propagated by recruitment of guanine exchange factors controlling the ras GTPase activity and phosphatidylinositol-3-OH kinase (PI3K) (4, 5). The well-characterized essential effector of PI3K in growth factor signaling is the Akt kinase, also known as PKB (protein kinase B).
Activation of PI3K results in accumulation of phosphatidylinositol-3,4,5-triphosphates (PIP3s) which initiates recruitment of Akt to the plasma membrane through its plekstrin homology domain. At this location Akt is phosphorylated on the Thr-308 and Ser-473 sites required to fully activate Akt (6). The activation loop of Akt on Thr-308 is phosphorylated by the phosphoinositide-dependent kinase 1 (PDK1) and required for the kinase activity of Akt (7–9). The Ser-473 kinase of Akt, named PDK2, which was sought for many years, was only recently identified as the mTOR Complex 2 (mTORC2) (10). Although several candidates were proposed earlier as PDK2 (9, 11), mouse genetic studies confirm the role of mTORC2 as the main Ser-473 kinase of Akt (12–15).
Originally, mTOR was discovered as a target for the lypophilic macrolide rapamycin. Rapamycin is well known as a potent immunosuppressant, as a potential anti-cancer drug, and also for effectively preventing restenosis after angioplasty. All these anti-proliferative effects of rapamycin are related to its specific targeting and inhibition of mTOR, a key player of an essential and conserved signaling pathway (16). The biochemical studies identify mTOR as a central component of two distinct and large protein complexes that play different roles in cells (17). Besides mTOR, mLST8 and DEPTOR have been identified as the mTOR interacting proteins within both complexes. A small adaptor protein mLST8 contains seven WD40 repeats, it binds tightly to the kinase domain of mTOR (18, 19) and is required for its kinase activity (20). The recently identified DEPTOR, the DEP domain containing TOR interacting protein, has been identified as a negative regulator of mTOR (21).
Binding of raptor to mTOR defines assembly of the first complex, known as mTORC1. This complex functions as a nutrient-sensitive kinase complex that regulates protein synthesis by phosphorylating its two substrates, S6K1 and 4EBP1. Rapamycin in a complex with its intracellular receptor FKBP12 specifically binds the FKBP12/rapamycin binding (FRB) domain on mTOR and inhibits the mTORC1 function (22). The FRB domain, a stretch of 100 amino acids, is located at the C-terminal half of mTOR. The mTOR kinase domain follows FRB domain, it is structurally resembles a kinase domain of PI3K but functions as a serine/threonine protein kinase and it is essential for mTOR’s function (16).
The second complex of mTOR, mTORC2, has been initially identified as a regulator of PKCa and cytoskeleton (18, 19). This signaling complex is assembled by mTOR and its essential components rictor and Sin1 (13, 18, 19, 23). Rictor and Sin1 form a heterodimer that determines mutual stability of both proteins. Rictor remains poorly characterized. The human rictor polypeptide contains 1,708 amino acids but reveals no homology to any known functional domain or protein, although it is conserved in all eukaryotes (10, 18). Initially, rictor’s ortholog, pianissimo, was identified in Dictyostelium as a critical player in chemotaxis and cAMP signal relay (24). Another component Sin1 might provide more insights into the regulation and function of mTORC2. Two Sin1 functional domains have been proposed: the Raf-like Ras binding domain (RBD) and a pleckstrin homology domain (25). The RBD domain points out Ras as a potential up-stream effector of mTORC2, and localization of mTORC2 at the plasma membrane might depend on the plekstrin homology domain of Sin1. Like rictor’s, Sin1’s ortholog was initially identified as an important regulator of chemotaxis and in addition as a Ras interacting protein 3 in Dictyostelium (26, 27).
In contrast to mTORC1, mTORC2 does not bind the rapamycin/FKBP12 complex, suggesting that the FRB domain on mTOR that is responsible for the binding is not accessible on mTORC2. Nevertheless, prolonged rapamycin treatment causes an indirect effect on mTORC2 by inhibiting the assembly of this complex. In some cell types, mostly lymphoma cells, the prolonged rapamycin treatment causes inhibition of Akt because of a dramatic effect on the abundance of mTORC2 (28).
The mTORC2 complex carries an important regulatory role in growth factor signaling by mediating the regulation between PI3K and its crucial downstream effector Akt. The growth factor-dependent regulation of the mTORC2 kinase activity is not understood. The mTORC2 component rictor has been described as a phosphoprotein. In this study we have characterized the growth factor-dependent phosphorylation of rictor and its regulation.
Results
Identification of the growth factor –dependent rictor phosphorylation in cancer cells
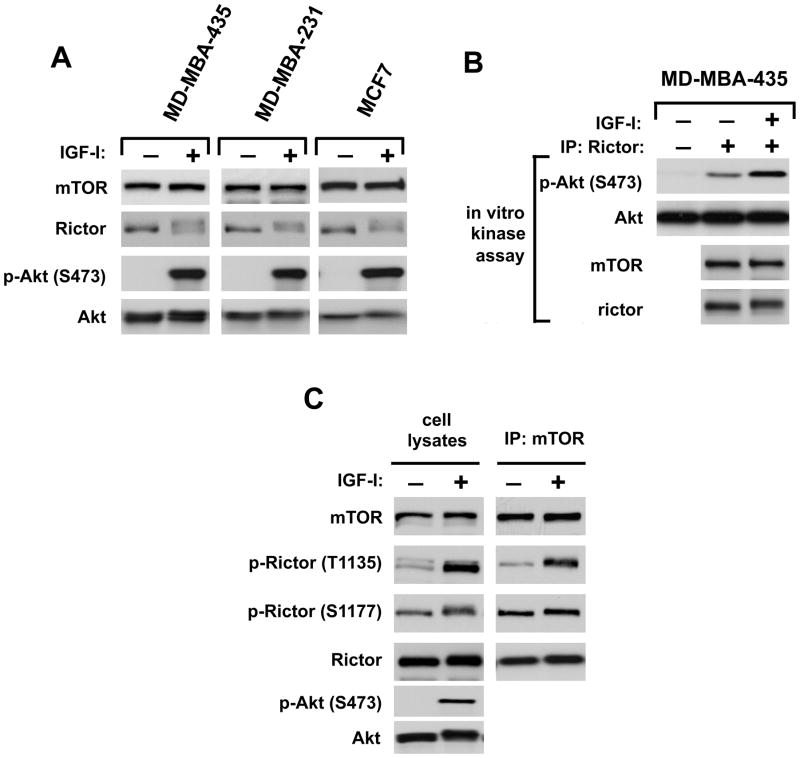
The kinase activity of mTORC2 as the Ser-473 regulatory kinase of Akt is dependent on growth factor signaling (10, 23). Rictor, as a component of mTORC2, has been initially characterized as a phosphoprotein (18). The growth factor-dependent phosphorylation of rictor has been studied in three cancer cell lines sensitive to the IGFI stimulation. We detected a robust phosphorylation of Akt on the Ser-473 site following the growth factor stimulation in the MDA-MB-435, MDA-MB-231, and MCF7 cells. We also found that the rictor’s protein mobility in a gel changed to a slower migratory form in the IGFI-stimulated compare to its mobility in the serum-starved cells (Fig. 1A). A slower migratory form of rictor has been shown to be dependent on its phosphorylation (18, 29). Activation of mTORC2 as the regulatory Akt kinase takes place following the stimulation of cells with IGFI as shown by performing the in vitro mTORC2 kinase assay (Fig. 1B), where mTORC2 has been purified by immunoprecipitation of rictor from the cellular lysates of MDA-MB-435 cells shown in Fig. 1A. These observations indicate that the growth factor-dependent activation of mTORC2 as the Ser-473 kinase of Akt is associated with the rictor phosphorylation.
FIGURE 1.
Rictor phosphorylation associates with the IGF-I dependent stimulation of the mTORC2 kinase activity. A. The IGFI-dependent rictor phosphorylation. The serum starved MDA-MB-231, MDA-MB-435 and MCF7 cells for 24 hrs were stimulated by IGF-I (40 ng/ml) for 20 min. The lysates were analyzed by immunoblotting for indicated proteins and phosphorylation of Akt. B. The mTORC2 kinase assay. The rictor immunoprecipitates from the serum starved or IGF-I stimulated MDA-MB-435 cells were used in a kinase assay with a full-length wild type Akt1 as a substrate. Immunoblotting was used to detect the phosphorylation of Akt on Ser-473 and the levels of mTOR, Rictor, and Akt1 in the kinase assay. In a first lane of the Akt immunoblots, Akt1 was used in a kinase assay to detect a basal phosphorylation of the substrate. C. Detection of the IGF-I dependent phosphorylation site of rictor. The serum starved MDA-MB-435 cells with or without the IGF-I stimulation were lysed and analyzed by immunoblotting for the indicated proteins and phosphorylation states by the phospo-specific rictor and Akt antibodies (left panel). In parallel, the mTOR associated rictor phosphorylation was analyzed in the mTOR immunoprecipitates as shown in a right panel.
We found that the growth factor-dependent regulation of the mTORC2 activity by IGFI is linked to phosphorylation of its component rictor, although the function of this phosphorylation in the mTORC2 signaling is unknown. To identify the rictor phosphorylation sites mass spectrometric analysis was carried out on the immunopurified rictor samples from the stimulated cells. Based on the alterations of mass spectrometry readings of the distinct rictor’s peptides, Thr-1135 and Ser-1177 have been identified with a high probability as rictor phosphorylation sites. To study a role of the rictor phosphorylation on this site, rictor phospho-specific antibodies have been developed. The specificity of the antibodies was validated by analyzing their binding to the immunopurified rictor proteins with the appropriate phospho-mutants. The phospho-specific antibodies did not recognize their mutated epitopes (Supplemental Data, Figure 2A). Most importantly, we found that the detection of rictor by the rictor phospho-Thr-1135 antibody was greater in the cellular extracts of MDA-MB-435 cells stimulated by IGFI than it was in the unstimulated serum-starved cells (Fig. 1C, a left panel). This finding confirmed that the rictor phosphorylation takes place on the Thr-1135 site following a stimulation of cells with IGFI. Whereas, the Ser-1177 site was most likely steadily phosphorylated, because it did not reveal a considerable change in the antibody binding following the IGFI stimulation.
After detecting rictor phosphorylation in total cellular lysates, we studied the phosphorylation of rictor within the mTORC2 complex. We purified mTORC2 by immunoprecipitating mTOR from cells in experimental conditions known to preserve the complex (18). We found that the mTORC2 component rictor was phosphorylated in response to the IGFI stimulation on the Thr-1135 site, but the phosphorylation on Ser-1177 was not affected (Fig. 1C, a right panel). In our following studies we focused only on the rictor Thr-1135 site, because we did not identified any conditions that regulate the Ser-1177 site. Thus, we found a similar pattern of the rictor phosphorylation in the total cellular extracts and the immunopurified mTORC2. The rictor phosphorylation in total cellular extracts represents the rictor phosphorylation within the mTORC2 complex and the phosphorylation state of Thr-1135 can be applied as the indirect activity marker of this kinase complex.
Deregulation of the rictor Thr-1135 phosphorylation by the oncogenic forms of ras and PI3K
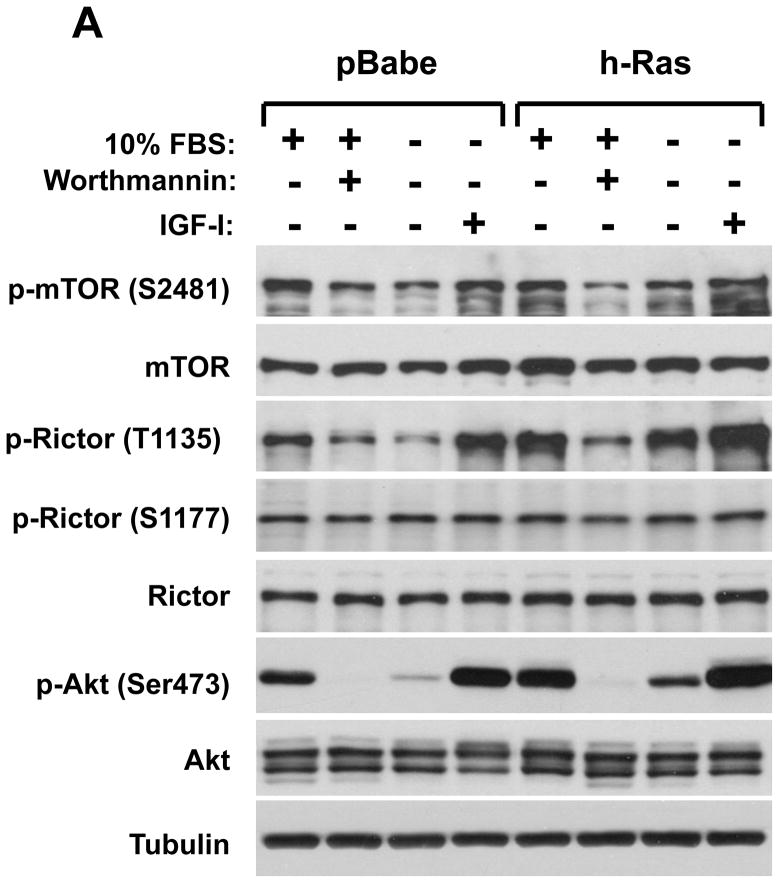
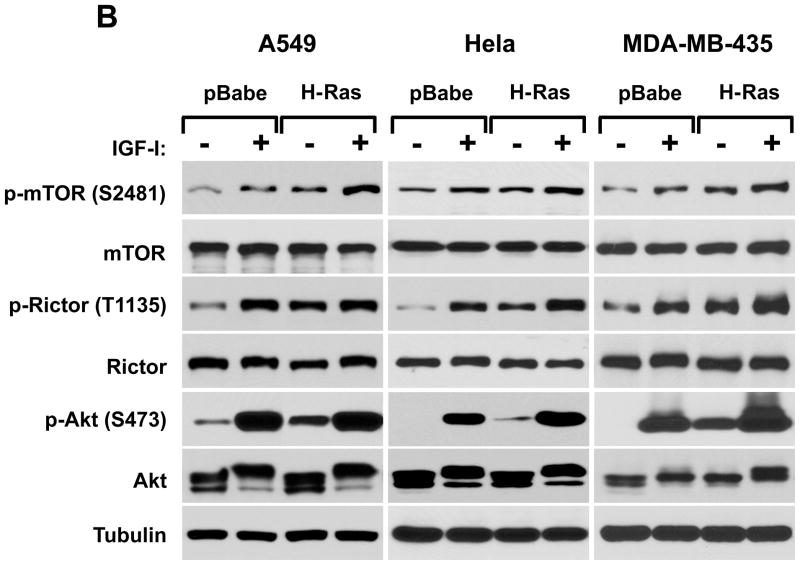
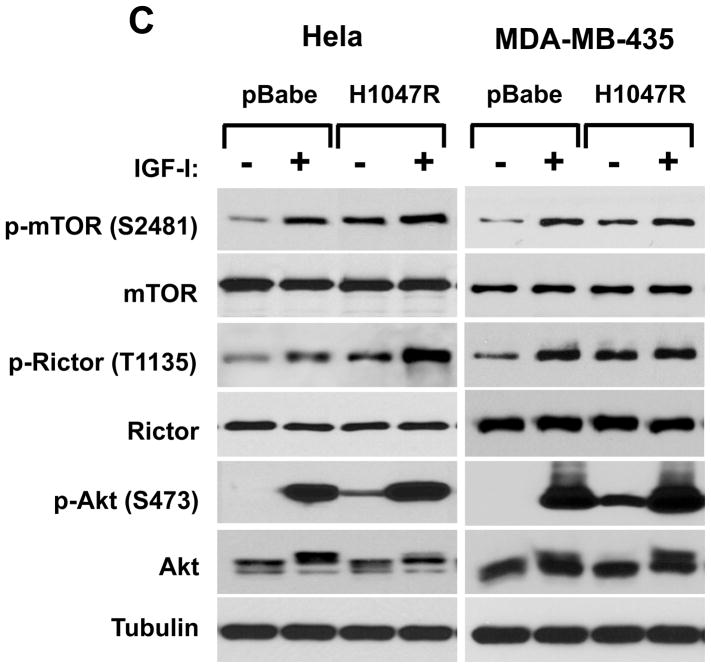
Our studies indicate that the rictor phosphorylation is dependent on growth factor signaling and PI3K pathway. It is known that some oncogenes cause constitutive activation of this important signaling pathway that gives an advantage for cancer cells in proliferation and survival (5, 30). The small GTPase Ras couples growth factor receptor signaling to ERK (MAPK) and PI3K pathways. The constitutively active form of ras is known as a common and potent oncogene that causes growth factor independent activation of the PI3K pathway (31, 32). In our next study we analyzed the effect of the ras oncogene on the phosphorylation of rictor on the Thr-1135 site in the non-tumorigenic MCF-10A human breast epithelial cell line (Fig. 2A). Constitutive expression of the oncogenic H-ras in MCF10A cells increased the basal phosphorylation level of rictor on the Thr-1135 site in cell growth culture conditions (medium containing 10 % serum) and also following stimulation by IGFI. In H-ras expressing cells this phosphorylation remained highly sensitive to PI3K inhibition by wortmannin, but most importantly, it was resistant to serum starvation. The rictor phosphorylation was similar to the Akt Ser-473 phosphorylation indicating that the rictor Thr-1135 site is a matching read-out of the PI3K-dependent mTORC2 activity. This finding is also supported by the mTOR Ser-2481 phosphorylation, a known mTORC2 activity marker. This study was extended into three different growth factor dependent cancer cell lines (A549, HeLa, and MDA MB-435) and revealed similar results (Fig. 2B). In addition to the ras oncogene, constitutive expression of the oncogenic form of PI3K also caused up-regulation of the Akt pathway that was associated with the hyperphosphorylation of rictor and mTOR in serum starved and growth factor stimulated cell culture conditions (Fig. 2C). It points out that activation of the PI3K activity is sufficient for activation of the mTORC2 signaling as evaluated by both mTORC2 activity markers.
FIGURE 2.
The mTORC2 signaling up-regulation by the ras or PI3K oncogenes is associated with the rictor T1135 phosphorylation. A loss of the growth factor-dependent rictor phosphorylation caused by the oncogenic form of H-ras. The oncogenic form of H-ras and empty vector as a control have been constitutively expressed in the non-tumorigenic MCF10A cells (A) or in A549, Hela, and MDA-MB-435 cells (B) based on the retroviral expression system. The transduced cells were selected and following the indicated treatments were lysed and analyzed by immunoblotting for the indicated proteins and phosphorylation states by the phospo-specific rictor, mTOR and Akt antibodies. As indicated, cells were growing in 10% serum with or without Wortmaninn at 100 nM concentration for 1 hour. In parallel, cells were serum starved for 24 hr or stimulated by IGFI (40 ng/ml) for 30 min. C. Expression of the oncogenic PI3K is sufficient in stimulation of the rictor Thr-1135 phosphorylation. The constitutively active form of the PI3K catalytic unit p110 (H1047R) has been constitutively expressed in A549, Hela and MDA-MB-435 cells based on the retroviral pBabe expression vector. The pBabe empty vector was used as control. The transduced and selected cells were starved for 24h and stimulated or not with IGF for 30 min at the concentration of 40 ng/ml.
Regulation of the rictor Thr-1135 phosphorylation
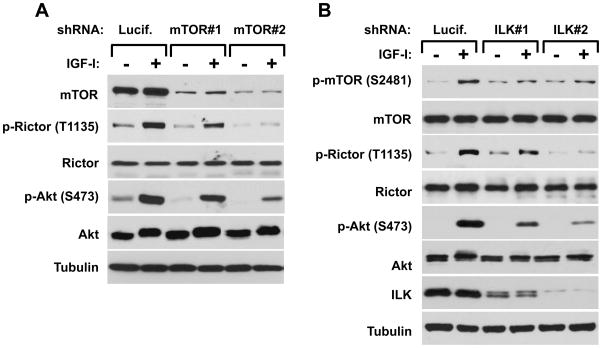
Within the known components of the mTORC2 complex, only mTOR carries the functional kinase domain (16, 17). In our next experiment we addressed whether the rictor Thr-1135 phosphorylation is dependent on mTOR. A partial knock down of mTOR by the shRNA#1 detects a weak effect on the phosphorylation of Akt that also inhibits partially the rictor Thr-1135 phosphorylation. The second mTOR shRNA expression carried a stronger effect on expression of mTOR and also stimulation of Akt that also caused a dramatic effect on the rictor phosphorylation (Fig. 3A). These data indicate that mTOR as a central component of both mTOR complexes controls the rictor Thr-1135 phosphorylation.
FIGURE 3.
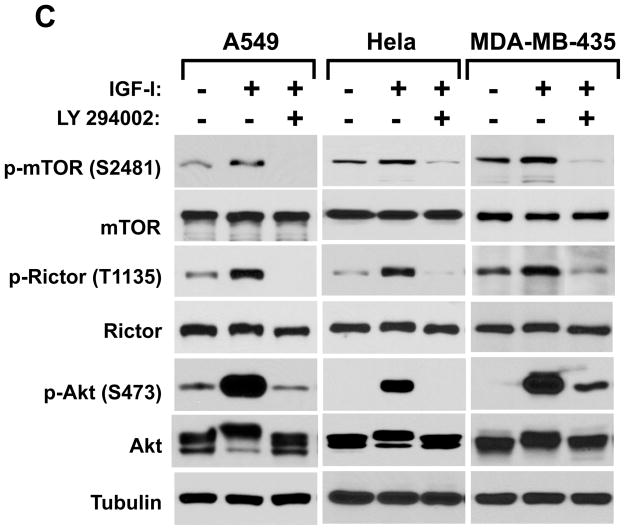
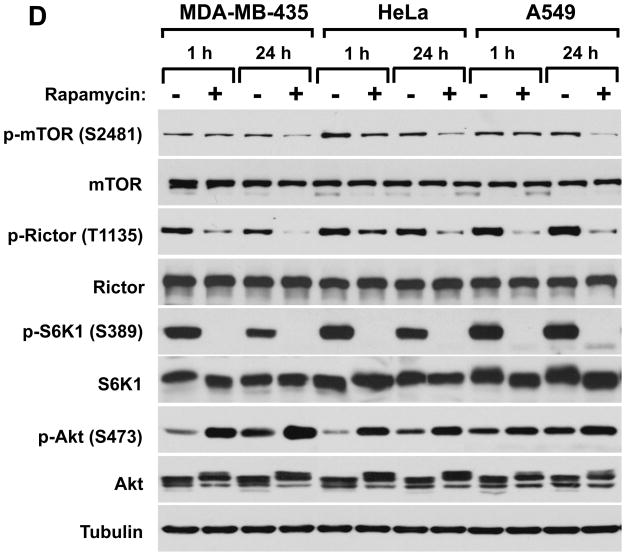
Regulation of the rictor T1135 phosphorylation. A. The phosphorylation of rictor on the Thr-1135 site is mTOR dependant. MDA-MB-435 cells were infected with lentiviruses expressing shRNA targeting luciferase (control) and mTOR. Following a serum starvation for 24 hrs and stimulation by IGFI (40 ng/ml) for 30 min. The cells were lysed and the equal amount of protein lysates were analyzed by western blotting by the indicated antibodies. B. The ILK knockdown inhibits the phosphorylation of rictor on the Thr-1135 site. MDA-MB-435 cells were infected with lentiviruses expressing shRNA targeting luciferase (control) and ILK. Following a serum starvation for 24 hrs and stimulation by IGFI (40 ng/ml) for 30 min. C. The growth factor-dependent phosphorylation of rictor is prevented by inhibition of PI3K. The MDA-MB-435, A549 and Hela cells were serum starved for 24h. Where indicated, cells were pre-incubated or not with the PI3-kinase inhibitor LY294002 at 50 mM concetration for 1 hour and stimulated with IGF-I (40 ng/ml) for 30 min. D. The rictor phosphorylation is rapamycin dependent. The MDA-MB-435, A549 and Hela cell lines were grown in the growth medium containing 10% serum with or without rapamycin at 100 nM concentration for the indicated periods of time.
In addition to the PI3K pathway, the inegrin linked kinase (ILK) has been characterized as an important regulator of Akt (33–35). Moreover, ILK has been shown to directly interact with rictor (36). In our study, we found that both rictor Thr-1135 and mTOR Ser-2481 phosphorylation sites are dependent on ILK (Fig. 3B). Although the ILK knock down was toxic in the studied cells, the levels of rictor and mTOR were not affected. In accordance with the previous studies (34, 35), we detected a low phosphorylation of Akt in the ILK knocked down cells stimulated by growth factor. Under these conditions, we found that the rictor and mTOR phosphorylation were inhibited. We observed that more efficient knock down of ILK had a stronger impact on the rictor but not on mTOR phosphorylation. This implies that compared to the mTOR Ser-2481 site, the rictor Thr-1135 site is more sensitive to ILK inhibition.
Recently, the hydrophobic Ser-2481 site of mTOR has been identified as an mTORC2 activity marker (37). We also found the mTOR phosphorylation on Ser-2481 only in the IGFI stimulated mTORC2 but not on mTORC1. In contrast, the mTOR Ser-2448 site was phosphorylated on both complexes (Supplementary Data, Fig. 3). Our study identified the rictor Thr-1135 phosphorylation site as a new activity marker of mTORC2. In our following experiments we analyzed regulation of the rictor Thr-1135 phosphorylation in parallel with the mTOR Ser-2481 site. First, we addressed whether the growth factor-dependent rictor phosphorylation is dependent on PI3K activity. We observed that in three cancer cell lines, pre-incubation of cells with the PI3K inhibitor LY 294002 completely abrogated the IGFI induced phosphorylation not only of Akt on Ser-473, but also rictor on Thr-1135 and mTOR on Ser-2481 (Fig. 3C). This indicates that similar to the mTOR Ser-2481 site, the rictor Thr-1135 phosphorylation site is dependent on the PI3K pathway and linked to the activity of mTORC2.
It is known that mTORC2 is sensitive to prolonged rapamycin treatment that leads to inhibition of the mTORC2 assembly and rictor phosphorylation (28, 29). In our next experiment, we studied the effects of short (1 hr) and long-term (24 hr) rapamycin treatments on rictor phosphorylation in three human cancer cell lines. Rapamycin by targeting mTORC1 inhibits activity of S6K1 as detected by its dephosphorylation on the Thr-389 site (Fig. 3D). Elimination of the negative feed back loop on the PI3K pathway by inhibiting the rapamycin-dependent S6K1 kinase is known to associate with the up-regulation of a basal PI3K-dependent phosphorylation of Akt on Ser473 (38–40), as we detected following the 1 hr and 24 hr rapamycin treatments. A decrease of the mTOR Ser-2481 phosphorylation has been detected only at the prolonged but not short-term rapamycin treatment that correlates well with the indirect impact of rapamycin on mTORC2. Interestingly, the rictor phosphorylation on Thr-1135 was sensitive to rapamycin within the short-term (1 hr) rapamycin treatments (Fig. 3D). Our finding indicates that the rictor Thr-1135 phosphorylation is sensitive to acute rapamycin treatment. In contrast to mTORC1, mTORC2 is not directly targeted by the rapamycin/FKBP12 complex suggesting insensitivity of mTORC2 to an acute rapamycin treatment (28). One potential mechanism of this regulation might be linked to the mTORC1-dependent phosphorylation of the rictor Thr-1135 site that points out on S6K1 as a kinase candidate. Indeed, three groups have recently reported that the rictor Thr-1135 site is phosphorylated by S6K1 (41–43) that explains a sensitivity of this site to an acute treatment of rapamycin.
Rictor is an essential component of mTORC2. Our initial characterization of the rictor Thr-1135 phosphorylation site indicates that this site is highly regulated and also dynamic in response to the cell growth conditions. We found that similar to the mTOR Ser-2481 site, the rictor phosphorylation is dependent on growth factor signaling pathway. This site is responsive to growth factor stimulation, PI3K activity, rapamycin, and expression of mTOR or ILK. The only difference we found in the behavior of both these sites is a sensitivity of the rictor Thr-1135 site to the short-term (1 hr) rapamycin treatment, whereas inhibition of the mTOR Ser-2481 site is detected only following a prolonged (24 hr) treatment.
Functional studies of the Thr-1135 site by mutagenesis
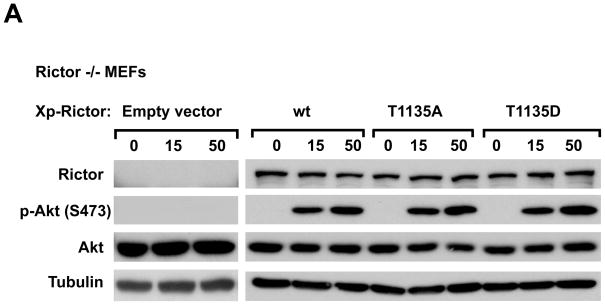
Following the confirmation of rictor phosphorylation on the Thr-1135 site, we studied its functional role on the growth factor-dependent activation of mTORC2. In pursuing this task, we developed the phospho-specific rictor mutants by substituting the phospho-sites to alanine or aspartic acid. To eliminate any interference from the endogenous rictor, the wild type and phospho-mutants rictor isoforms were expressed in the rictor null mouse embryonic fibroblasts (MEFs) described previously (12). In the rictor null cells, we did not detect any traces of rictor expression and the growth factor-dependent phosphorylation of Akt on the Ser-473 site (Fig. 4A). The wild type rictor expression in these cells reconstituted the rictor-dependent function of mTORC2 as the growth factor-dependent Ser-473 kinase of Akt. Following the IGFI stimulation of the transfected cells, the phosphorylation of Akt on the Ser-473 site occurred in a growth factor-dependent manner. In parallel, similar levels of the phospho-rictor mutants were expressed and their effect on the regulation of Akt was assessed. Prevention of the rictor Thr-1135 phosphorylation by a substitution of this site to alanine did not alter the mTORC2-dependent phosphorylation of Akt in the MEFs cells. At the same time, mimicking a constitutive phosphorylation of rictor on Thr-1135 by mutating this site to aspartic acid did not cause any detectable changes on the phosphorylation of Akt (Fig. 4A). We also did not detect any effect on the phosphorylation of Akt by expressing the phospho-Ser-1177 rictor mutants (Supplementary Data, Fig. 2B). The double rictor mutant preventing the phosphorylation of both Thr-1135 and Ser-1177 sites also did not have a substantial effect on regulation of Akt (data not shown). These data indicated that the mutations on the identified rictor phosphorylation sites did not carry a substantial effect on the growth factor-dependent activation of mTORC2 that correlates well with the recent report by Treins et al. on the functional characterization of the rictor Thr-1135 site (43).
FIGURE 4.
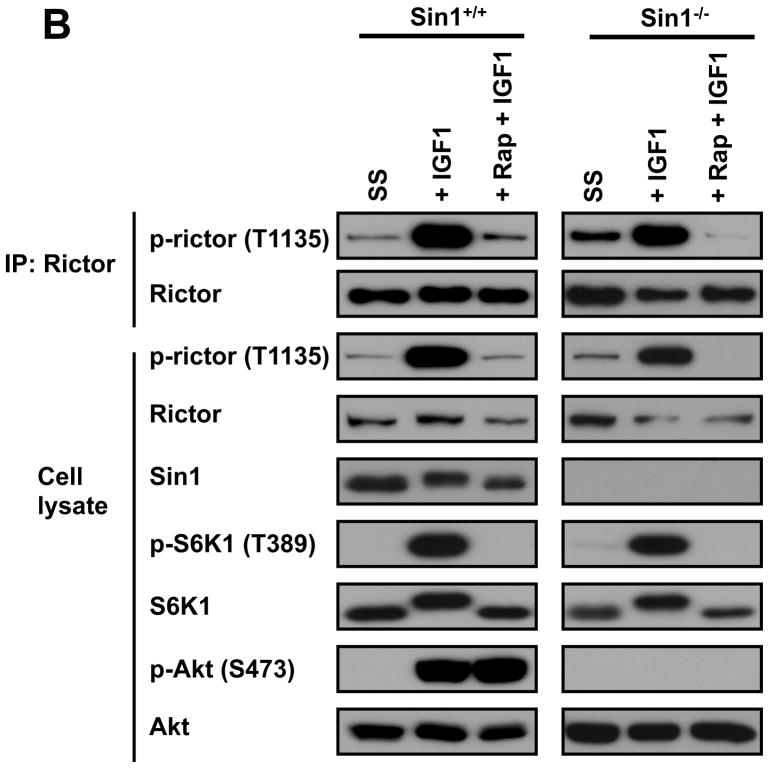
Characterization of the rictor Thr-1135 phosphorylation. A. The functional study of the rictor T1135 phosphorylation site. Reconstitution of the mTORC2 signaling in rictor null MEFs by expressing the wild type and phospho-rictor mutants (T1135A and T1135D). Following 48 hrs after transfection cells were lysed and cell extracts analyzed by immunoblotting for the indicated proteins and phosphorylation state of Akt on the Ser-473 site. B. The rapamycin-sensitive rictor phosphorylation does not require mTORC2. The wild type and Sin1 KO MEFs were serum starved overnight. As indicated the cells were stimulated by IGFI (50 ng/ml) for 20 min or treated with rapamycin (50 ng/ml) for 15 min prior stimulation of cells with the growth factor. The cells were lysed and analyzed as described in panel A.
Our data described in Fig. 4A indicate that the phosphorylation of rictor on Thr-1135 site is not essential in regulation of growth factor-dependent regulation of the mTORC2 activity as the regulatory kinase of Akt. If this rictor phosphorylation is dispensable in regulation of mTORC2, this modification of rictor might play a role in the mTOR-independent function of rictor. In our next experiment we studied whether rictor phosphorylation on Thr-1135 occurs in the mTORC2-deficient cells. Similar to rictor, Sin1 is also required for the assembly and functional integrity mTORC2. The Sin1 KO cells have been characterized as the mTORC2 deficient cells, where rictor without its binding partner Sin1 becomes an unstable protein and does not bind mTOR (13). To attain the comparable levels of rictor in wild type and Sin1 KO MEFs, the mutant’s total protein lysate concentration was increased two times. We found that in wild type MEFs following the IGFI stimulation both mTORC1 and mTORC2 were responsive to growth factor as detected by phosphorylation of their substrates S6K1 and Akt (left panel, Fig. 4B), where only the mTORC1-dependent phosphorylation of S6K1 was detected in Sin1 KO cells (right panel, Fig. 4B). Importantly, in both cell types we have detected the growth factor dependent phosphorylation of rictor on the Thr-1135 site as shown by analysis of the immunopurified rictor or total cell lysates. This rictor phosphorylation was associated with the mTORC1-dependent activation of S6K1 and was highly sensitive to rapamycin (Fig. 4B). Our study indicates that the rapamycin-dependent rictor The-1135 phosphorylation does not require assembly of rictor to the mTORC2 complex or its interaction with Sin1 and implies a role of this phosphorylation in regulation of the mTORC2-independent function of rictor that remains to be elucidated.
Discussion
The mTORC2 signaling complex functions as the regulatory Ser-473 kinase of Akt, therefore the main known function of this kinase complex is to mediate a signaling between PI3K and its important downstream effector Akt. How growth factor signaling regulates mTORC2 is not known and in this study we show that activation of mTORC2 is coupled to a specific phosphorylation of its component rictor. Initially, rictor was identified as a phosphoprotein, later the rapamycin-dependent phosphorylation of rictor was also reported (18, 29). These studies were based on changes of the rictor protein mobility in a gel. Previously, specific rictor phosphorylation sites have not been identified and characterized. In this report, we demonstrate that rictor is specifically phosphorylated on the Thr-1135 site and that this phosphorylation is coupled to the growth factor-dependent activation of mTORC2.
Based on our characterization of the rictor Thr-1135 phosphorylation, we identified this phosphorylation is coupled to growth factor signaling and is also a rapamycin dependent site of rictor. First, similar to mTORC2 activity and its substrate Akt phosphorylation, this rictor site is dephosphorylated in serum starved cells and is highly responsive to growth factor stimulation. Second, the oncogenic factors activating the ras/PI3K pathway and mTORC2 signaling cause the growth factor-independent phosphorylation of rictor on Thr-1135. Third, the rictor phosphorylation is similar to the mTORC2-dependent Akt phosphorylation as being sensitive to inhibition of PI3K, mTOR, and ILK. Moreover, the identified regulation of the rictor Thr-1135 phosphorylation correlates well with the mTOR Ser-2481 phosphorylation which was recently identified as an mTORC2 activity marker (37).
We have shown that expression of the oncogenic form of ras or PI3K caused the growth factor-independent activation of the mTORC2 signaling. Earlier, it has been proposed that activation of mTORC2 might take place by a binding of ras to the putative ras binding domain on the mTORC2 component Sin1 (13, 23). We found that the ras induced up-regulation of the mTORC2 activity is highly sensitive to inhibition of PI3K. This finding indicates that the ras-dependent activation of mTORC2 is PI3K dependent suggesting insufficiency of the constitutively active form of ras in a direct activation of mTORC2. Ras is a critical intermediate in coupling a signaling between growth factor receptor and PI3K, whereas the ras binding domain of PI3K is required for ras-driven tumorigenesis (44). This binding domain might function as a link in growth factor- and ras-dependent activation of mTORC2.
Prolonged rapamycin treatment inhibits assembly of mTORC2 and we detected that both mTORC2 phospho-markers, rictor Thr-1135 and mTOR Ser-2481 sites, are sensitive to the long-term (24 hr) treatment of rapamycin. This effect of the drug well correlates with the reported long-term effect of rapamycin on the mTORC2 assembly (28). Surprisingly, the rictor Thr-1135, but not the mTOR Ser-2481, phosphorylation site was affected within the short-term (1 hr) rapamycin treatment. It is a first observation describing the effects of rapamycin on mTORC2 within a short-term treatment. In the previous work, it has been proposed that only prolonged, but not short-term, rapamycin treatment affects mTORC2 by interfering with the assembly of this complex (28). It is possible that the rictor Thr-1135 phosphorylation is dependent on mTORC1 that is known to be a directly targeted by rapamycin. This finding is coherent with the recent reports from three laboratories that identified the mTORC1 substrate S6K1 as a kinase of rictor on the Thr-1135 site (41–43). This finding indicates that activation of the mTORC1/S6K1 signaling coordinates a rictor function by phosphorylating it specifically at least within one site that explains a high sensitivity of this rictor phosphorylation to rapamycin.
In this study we addressed a role of the rictor Thr-1135 phosphorylation on activation of mTORC2 by transiently expressing the wild type rictor and its phospho-specific mutants in the rictor null cells. We did not detect any substantial effect of the rictor phospho-mutants on the mTORC2 signaling. It is possible that a constitutive expression of rictor and its mutants will be appropriate for a long-term analysis of the role of these phospho-mutants on mTORC2 and will lead to the understanding of the functional role of this phosphorylation site. Our study of the rictor Thr-1135 phosphorylation site revealed that the alteration of this site is not sufficient to affect the growth factor-dependent activation of the mTORC2 signaling that correlates well with the report from the Downward group (43). The reports from the Manning and Roux groups (41, 42) indicated a weak stimulation of the mTORC2 function by a substitution of rictor by the T1135A phospho-mutant as detected by the Akt phosphorylation. One potential explanation in this variation of the phospho-rictor mutant studies might be linked to application of the different rictor KO MEFs. The spontaneously immortalized rictor KO MEFs (12) have been studied by the Downward group and in our work, whereas the Manning and Roux groups have applied the double rictor and p53 KO MEFs (15). Nevertheless, the rictor Thr-1135 phosphorylation site is conserved in vertebrates and most likely carries an important role in regulation of mTORC2 by providing the phosphorylation-dependent binding site to 14-3-3 proteins (41, 43).
The recent reports characterizing the rictor Thr-1135 phosphorylation have been focused on its role in regulation of the mTORC2 function. We found that the rictor Thr-1135 phosphorylation takes place not only within the rictor assembled within the mTORC2 complex but also in the mTORC2 deficient cells. We believe that rictor also carries the mTORC2 independent function that is supported by the work describing a role of rictor in regulation of cell morphology by binding to unconventional myosin Myo1c (45). It has been described that the mTOR-independent binding of rictor to Myo1c is independent on growth factor signaling suggesting that the rictor Thr-1135 phosphorylation is not involved in regulation of the rictor and Myo1c interaction.
In summary, we show that following growth factor stimulation and activation of the PI3K/mTOR/Akt pathway, the mTORC2 component rictor is specifically phosphorylated on the Thr-1135 site. This phosphorylation is controlled by the mTORC1/S6K1 signaling and is highly sensitive to rapamycin. A regulation of the mTORC2 function by the mTORC1 signaling through the phosphorylation of rictor by S6K1 is an attractive regulatory mechanism in coordination of the two branches of the mTOR pathway. Under the experimental settings presented in this work the rapamycin-dependent rictor phosphorylation was not essential in regulation of the mTORC2 function as the regulatory kinase of Akt. We found that the rictor Thr-1135 phosphorylation takes place in the mTORC2-deficient cells implying a role of this phosphorylation in regulation of the mTORC2-independent rictor function that remains to be characterized.
Materials and Methods
Materials
Reagents were obtained from the following sources: protein G-Sepharose from Pierce; rapamycin and LY294002from LC Laboratories; DMEM/F12 from Life Technolo gies; insulin-like growth factor I from Peprotech; the Fetal Bovine Serum (FBS) from Hyclone, Fugene 6 transfection reagent and the complete protease inhibitor cocktail from Roche; DramFect transfection reagent from OZ Biosciences; the antibody to Xpress tag from Invitrogen; the antibodies to phospho-T1135 and phospho-S1177 rictor, phospho-S2448 and phospho-S2481 mTOR, mTOR, Raptor, S6K1, phospho-T389 S6K1, phospho-S473 and phospho-T308 Akt/PKB, Akt from Cell Signaling Technologies; the antibodies to Rictor, HRP-labeled anti-rabbit, anti-mouse, anti-goat secondary antibodies, and tubulin from Santa Cruz Biotechnology. Lentiviral shRNAs targeting human mTOR were generated and used as described previously. ILK shRNAs (shRNA) were obtained from Sigma (MISSION ® shRNA). The retroviral expression plasmids of H-ras (plasmid#18749, pWZL hygro H-ras V12) and PI3K H1047R (plasmid#12524, pBABE puro PI3KCA H1047R) were obtained from Addgene.
Cell lines and culture
A549, Hela, MDA-MB-435 and MCF10A were obtained from American Type Culture Collection. MEFs, A549, Hela, and MDA-MB-435 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 with 10% FBS. MCF10A cells were cultured in DMEM/F12 supplemented with insulin 10 μg/ml, epidermal growth factor 20 ng/ml, and hydrocortisone 0.5 μg/ml, cholera toxin 100 ng/ml, CaCl2 1 mM, and horse serum 5%. All of the above cell lines were cultured at a density that allowed cell division throughout the course of the experiment. When indicated cells were treated with 100 nM rapamycin in growth medium containing 10% FCS for the indicated time. For LY294002 experiments, cells of 60–70% confluency were serum starved for 24 hours and incubated with 50 mM LY294002 for 1 hour prior the stimulation by Insulin-like Growth Factor I (IGFI) 40 ng/ml for 30 min. Mouse embryonic firoblasts were transfected by the DramFect reagent following the manufacture protocol.
The rictor mutagenesis
The rictor cDNA from the pRK5 plasmid was re-subcloned to pcDNA4 with the Xpress tag by using pcDNA4/HisMax TOPO TA Expression Kit from Invitrogen. A large size of the plasmid (about 11 kb) interfered with the PCR-based mutagenesis. To decrease a size of the plasmid to 6 kb size, the fragment of rictor containing a sequence of the phospho-mutants have been subcloned to pBluescript by BglII and NotI digestion. The primers for mutagenesis were designed based on the QuickChange Primer Design Program available at the Stratagene’s website. The pBluescript plasmid containing the rictor fragment has been mutagenized with the QuickChange XLII mutagenesis kit (Stratagene). Following the validation of mutations by sequencing, the rictor mutated fragments were reintroduced back to the Xp-rictor pcDNA4 plasmid by the BglII and NotI digestion.
Cell lysis and immunoblotting
All cells were rinsed with ice-cold PBS before lysis in buffer containing (40 mM HEPES [pH 7.5], 120 mM NaCl, 1 mM EDTA, 10 mM Na-pyrophosphate, 10 mM sodium glycerophosphate, 50 mM NaF, 1% Triton X-100 and protease inhibitor cocktail (Roche). The scraped lysates were incubated for 20 min at 4°C to complete lysis. The soluble fractions of cell lysates were isolated by centrifugation at 13,000 rpm for 10 min in a microcentrifuge. Samples of cellular lysates containing the equal total protein concentration were resolved by SDS-PAGE. Resolved proteins on a gel were transferred to PVDF membranes and were visualized by immunoblotting as described previously (28).
Mass spectrometric analysis of rictor
In-gel digestions were performed according to standard protocols. For this analysis rictor was purified on a gel by combining ten rictor immunoprecipitation samples, the protein was visualized by Coomassie staining. Briefly, excised rictor gel bands were washed three times with 50% acetonitrile/200mM ammonium bicarbonate. Reduction was performed with 10 mM DTT in 100 mM ammonium bicarbonate at 60 °C for 30 minutes and alkylation was performed with 20 mM iodoacetamide in 100 mM ammonium bicarbonate at room temperature for 30 minutes in the dark. Digestion with trypsin (1.0 ug enzyme added, Promega, Madison, WI) was performed at 30 °C overnight. Peptides were extracted from the gel bands 3 times with 60% acetonitrile in 0.1% trifluoroacetic acid at 30 °C for 30 minutes, and the volume was reduced to 10 uL by vacuum centrifugation.
Nano-LC/MS/MS was performed on a LTQ linear ion trap mass spectrometer (Thermo Electron Corporation) coupled with an 1100 series nanoLC system (Agilent Technologies). The nano-LC column was a 75 um ID × 360 um OD PicoFrit column (New Objective, Woburn, MA) packed with 3 um Magic C18 resin (Michrom Bioresources, Auburn, CA). Mass spectra were acquired over a ninety minute gradient (A: 0.1 % formic acid; B: 90 % acetonitrile in 0.1 % formic acid) by data dependent acquisition in which the top eight most intense ions per MS scan (mass range of 300-2000 m/z) were selected for collision induced disassociation MS/MS. Dynamic exclusion was enabled with a repeat count of 2, repeat duration of 0.5 minutes, and exclusion duration of 1.5 minutes. MS/MS spectra were analyzed using a combination of Spectrum Mill (Agilent Technologies) and manual interpretation.
Immunoprecipitations and kinase assays
For immunoprecipitation experiments the lysis buffer contained 0.3% CHAPS instead of 1% Triton in order to preserve the integrity of the mTOR complexes. As described previously (10), 4 mg of rictor antibodies were added to the cleared cellular lysates (1 mg protein content in 700 ml) and incubated with rotation for 90 min. Following with 1 hr incubation with 20 ml of a 50% slurry of protein G-Sepharose. Immunoprecipitates captured with protein G-Sepharose were washed four times with the CHAPS containing lysis buffer and once with the rictor-mTOR kinase buffer (25 mM Hepes pH 7.5, 100 mM potassium acetate, 2 mM MgCl2). For kinase reaction, immunoprecipitates were incubated in a final volume of 15 ml for 20 min at 37°C in the rictor-mTOR kinase buffer containing 500 ng inactive Akt1-GST and 500 mM ATP. The reaction was stopped by the addition of 200 ml of the ice-cold Enzyme Dilution buffer (20 mM MOPS, pH 7.0, 1 mM EDTA, 0.3% CHAPS, 5% glycerol, 0.1 % 2-mercaptoethanol, 1 mg/ml BSA). After a quick spin, the supernatant was removed from the protein G-Sepharose, and a 15 μl portion was analyzed by immunoblotting for phospho-Ser473 Akt and Akt level detection. The pelleted G-Agarose beads were also analyzed by immunoblotting to determine the levels of rictor and mTOR in the immunoprecipitates.
Retroviral Vector, Retroviral Production, and Infection
The retroviral vectors used in as plasmids were propagated in and purified from XL-10 Gold bacterial cells and cotransfected with together with the Delta VPR and VSVG plasmids into actively growing cells as described previously (18). The day prior transfection, Human Embryonic Kidney (HEK) 293T cells (1.2 × 106) were plated on 6 cm plates in 3 ml DMEM supplemented with 10% fetal bovine serum. Retroviruses were harvested 48 hours after transfection and spinned at 3000 g for 15 min in order to eliminate any remaining HEK 293T cells. The day prior infection, cells to be infected were seeded in six-well dishes. The viral supernatant was added at a ratio of 1:1 to the culture medium in the presence of polybrene (8 mg/ml) and the cells were spinned at 1800 rpm for 45 min in order to increase the infection rate. Cells incubated with the retroviruses for the following 24 hours. A second infection was performed following the same protocol the next day. After an additional 24 hours of recovery in normal medium, infected cells were passaged and selected with puromycin (2.5 mg/ml for 3–4 days).
Supplementary Material
Acknowledgments
We greatly appreciate the work by the Cell Signaling Technologies team for development of the high quality phospho-specific rictor antibodies provided for this study. We also thankful to Dr. Bing Su for providing the Sin1 KO MEF cell lines. The work was supported by the and the M. D. Anderson Cancer Center Fellow Trust fund, The M. D. Anderson Cancer Center Breast SPORE, American Cancer Society (RSG-09-026-01CCG01), and NIH grant CA133522 to DDS and NIH grant AI104389 to DMS.
References
- 1.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 2.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–72. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 3.Schlessinger J, Lemmon MA. SH2 and PTB domains in tyrosine kinase signaling. Sci STKE 2003. 2003:RE12. doi: 10.1126/stke.2003.191.re12. [DOI] [PubMed] [Google Scholar]
- 4.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 5.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 7.Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 8.Stephens L, Anderson K, Stokoe D, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–4. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 9.Dong LQ, Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005;289:E187–96. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- 10.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 11.Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26:1338–45. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 12.Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–9. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–32. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE 2003. 2003:re15. doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- 17.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 19.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Sarbassov DD, Ali SM, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 21.Peterson TR, Laplante M, Thoreen CC, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–86. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 23.Frias MA, Thoreen CC, Jaffe JD, et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–70. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Chen MY, Long Y, Devreotes PN. A novel cytosolic regulator, Pianissimo, is required for chemoattractant receptor and G protein-mediated activation of the 12 transmembrane domain adenylyl cyclase in Dictyostelium. Genes Dev. 1997;11:3218–31. doi: 10.1101/gad.11.23.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroder WA, Buck M, Cloonan N, et al. Human Sin1 contains Ras-binding and pleckstrin homology domains and suppresses Ras signalling. Cell Signal. 2007;19:1279–89. doi: 10.1016/j.cellsig.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Parent CA, Insall R, Firtel RA. A novel Ras-interacting protein required for chemotaxis and cyclic adenosine monophosphate signal relay in Dictyostelium. Mol Biol Cell. 1999;10:2829–45. doi: 10.1091/mbc.10.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Comer FI, Sasaki A, et al. TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Mol Biol Cell. 2005;16:4572–83. doi: 10.1091/mbc.E05-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 29.Akcakanat A, Singh G, Hung MC, Meric-Bernstam F. Rapamycin regulates the phosphorylation of rictor. Biochem Biophys Res Commun. 2007;362:330–3. doi: 10.1016/j.bbrc.2007.07.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Lintig FC, Dreilinger AD, Varki NM, Wallace AM, Casteel DE, Boss GR. Ras activation in human breast cancer. Breast Cancer Res Treat. 2000;62:51–62. doi: 10.1023/a:1006491619920. [DOI] [PubMed] [Google Scholar]
- 31.Friday BB, Adjei AA. K-ras as a target for cancer therapy. Biochim Biophys Acta. 2005;1756:127–44. doi: 10.1016/j.bbcan.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Giehl K. Oncogenic Ras in tumour progression and metastasis. Biol Chem. 2005;386:193–205. doi: 10.1515/BC.2005.025. [DOI] [PubMed] [Google Scholar]
- 33.Persad S, Dedhar S. The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 2003;22:375–84. doi: 10.1023/a:1023777013659. [DOI] [PubMed] [Google Scholar]
- 34.Persad S, Attwell S, Gray V, et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276:27462–9. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- 35.Persad S, Attwell S, Gray V, et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci U S A. 2000;97:3207–12. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald PC, Oloumi A, Mills J, et al. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008;68:1618–24. doi: 10.1158/0008-5472.CAN-07-5869. [DOI] [PubMed] [Google Scholar]
- 37.Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–7. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 40.Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674–84. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 41.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009 doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol. 30:908–21. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treins C, Warne PH, Magnuson MA, Pende M, Downward J. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 29:1003–16. doi: 10.1038/onc.2009.401. [DOI] [PubMed] [Google Scholar]
- 44.Gupta S, Ramjaun AR, Haiko P, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–68. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 45.Hagan GN, Lin Y, Magnuson MA, Avruch J, Czech MP. A Rictor-Myo1c complex participates in dynamic cortical actin events in 3T3-L1 adipocytes. Mol Cell Biol. 2008;28:4215–26. doi: 10.1128/MCB.00867-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.