Table 1.
α-Tetrasubstituted α-amino acids stored in NCAD. The systematic name (and the common and abbreviated names,a when available), the chemical structure, and the reference reporting the quantum mechanical study are given for each amino acid.
| Abbreviated name |
Systematic name (common name) |
Structure | Ref. |
|---|---|---|---|
| Aib | 2-amino-2-methylpropanoic acid (α-aminoisobutyric acid) |
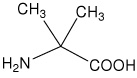 |
20 |
| Ac3c | 1-aminocyclopropanecarboxylic acid | 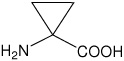 |
21 |
| (1S,2S)c3Phe | (1S,2S)-1-amino-2-phenylcyclopropanecarboxylic acid |
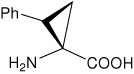 |
21 |
| (1S,2R)c3Phe | (1S,2R)-1-amino-2-phenylcyclopropanecarboxylic acid |
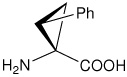 |
21 |
| l-c3Dip | (S)-1-amino-2,2-diphenylcyclopropanecarboxylic acid |
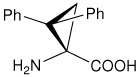 |
22 |
| (2S,3S)c3diPhe | (2S,3S)-1-amino-2,3- diphenylcyclopropanecarboxylic acid |
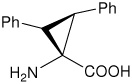 |
23 |
| Dpg | 2-amino-2,2-diphenylacetic acid (diphenylglycine) |
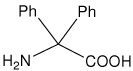 |
24 |
| Dbg | 2-amino-2-benzyl-3-phenylpropanoic acid (dibenzylglycine) |
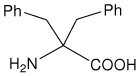 |
25 |
| Ac4c | 1-aminocyclobutanecarboxylic acid | 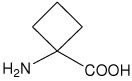 |
26 |
| Ac5c | 1-aminocyclopentanecarboxylic acid | 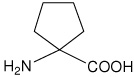 |
27 |
| Adt | 4-amino-1,2-dithiolane-4-carboxylic acid |
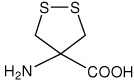 |
28 |
| (1S,2S)c5Phe | (1S,2S)-1-amino-2-phenylcyclopentanecarboxylic acid |
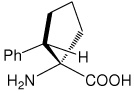 |
29 |
| (1S,2R)c5Phe | (1S,2R)-1-amino-2-phenylcyclopentanecarboxylic acid |
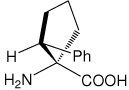 |
29 |
| (1S,2S)c5Arg | (1S,2S)-1-amino-2-(guanidinomethyl)- cyclopentanecarboxylic acid |
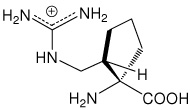 |
30 |
| (1S,2R)c5Arg | (1S,2R)-1-amino-2-(guanidinomethyl)- cyclopentanecarboxylic acid |
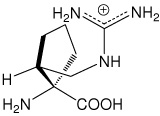 |
30 |
| Ac6c | 1-aminocyclohexanecarboxylic acid | 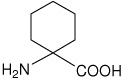 |
31 |
| (1S,2S)c6Phe | (1S,2S)-1-amino-2-phenylcyclohexanecarboxylic acid |
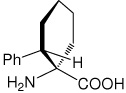 |
32 |
| (1S,2R)c6Phe | (1S,2R)-1-amino-2-phenylcyclohexanecarboxylic acid |
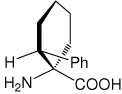 |
32 |
| Toac | 4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl-4- carboxylic acid |
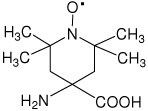 |
33 |
| — | (1S,2R,4R)-2-aminobicyclo[2.2.1]heptane-2- carboxylic acid |
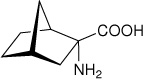 |
34 |
| — | (1R,2R,4S)-2-aminobicyclo[2.2.1]heptane-2- carboxylic acid |
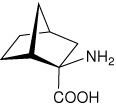 |
34 |
| — | (1S,2S,3R,4R)-2-amino-3- phenylbicyclo[2.2.1]heptane-2-carboxylic acid |
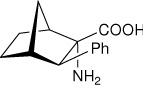 |
34 |
| — | (1S,2S,3S,4R)-2-amino-3- phenylbicyclo[2.2.1]heptane-2-carboxylic acid |
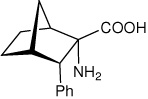 |
34 |
| — | (1R,2S,3R,4S)-2-amino-3- phenylbicyclo[2.2.1]heptane-2-carboxylic acid |
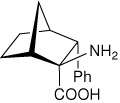 |
34 |
| — | (1R,2S,3S,4S)-2-amino-3- phenylbicyclo[2.2.1]heptane-2-carboxylic acid |
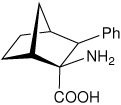 |
34 |
| l-(αMe)Pro | (2S)-2-methylpyrrolidine-2-carboxylic acid [(α-methyl)proline)] |
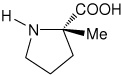 |
35 |
| l-(αPh)Pro | (2R)-2-phenylpyrrolidine-2-carboxylic acid [(α-phenyl)proline] |
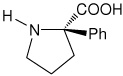 |
35 |
| — | (1S,2R,3R,5R,6R,7S,8R,9R,10R)-8- aminopentacyclo[5.4.0.0(2,6).0(3,10).0(5,9)]-undecane-8- carboxylic acid |
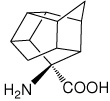 |
36 |
| — | (S)-4-aminopentacyclo[6.3.0.0(2,6).0(3,10).0(5,9)]- undecane-4-carboxylic acid |
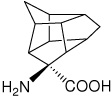 |
37 |
In the abbreviated name, the configuration is defined by the l/d nomenclature when only the α carbon is chiral (l usually corresponding to S). When two or more chiral centers are present, the R/S stereochemical descriptors are used instead.
