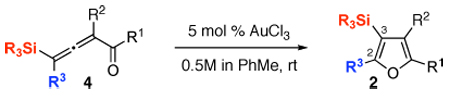
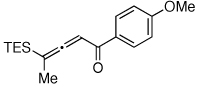
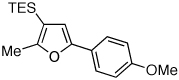
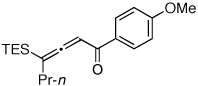
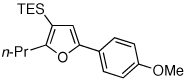
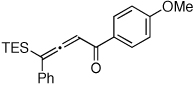
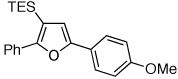
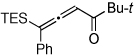
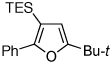
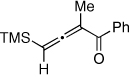
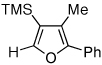
Table 1.
Au-Catalyzed Cycloisomerization of Allenyl Ketones 4
Isolated yield of product for reactions performed on 0.5 mmol scale.
Isolated yield of 2e for reaction performed in MeNO2.
Isolated yield of 2e for reaction performed with 1 mol % of catalyst.