Table 3.
Au-Catalyzed Cycloisomerization of Homopropargyl Ketones 6
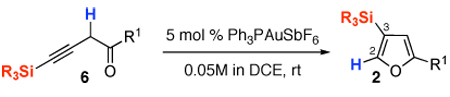 | |||
|---|---|---|---|
| Entry | Substrate | Product | 2, %a,b |
| 1 | 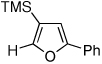 |
2f, 79 | |
| 2 | 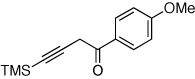 |
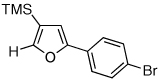 |
2g, 91 |
| 3 | 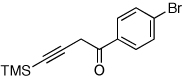 |
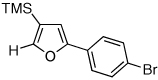 |
2h, 91c |
| 4 | 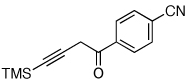 |
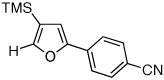 |
2i, 48d |
| 5 | 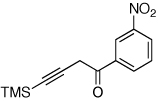 |
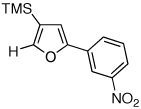 |
2j, 65e |
| 6 | 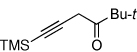 |
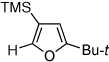 |
2k, 71f |
| 7 | 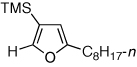 |
2l, 68 | |
| 8 | 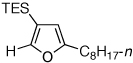 |
2m, 77 | |
| 9 | 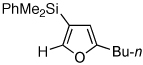 |
2n, 67 | |
Isolated yield of product for reactions performed on 0.5 mmol scale.
2f and 2l contained 8 and 6% of the corresponding desilylated furan, respectively.
14:1 ratio of 2h:3h.
10:1 ratio of 2i:3i.
8:1 ratio of 2j:3j.
GC Yield, 2k is a volatile compound.
