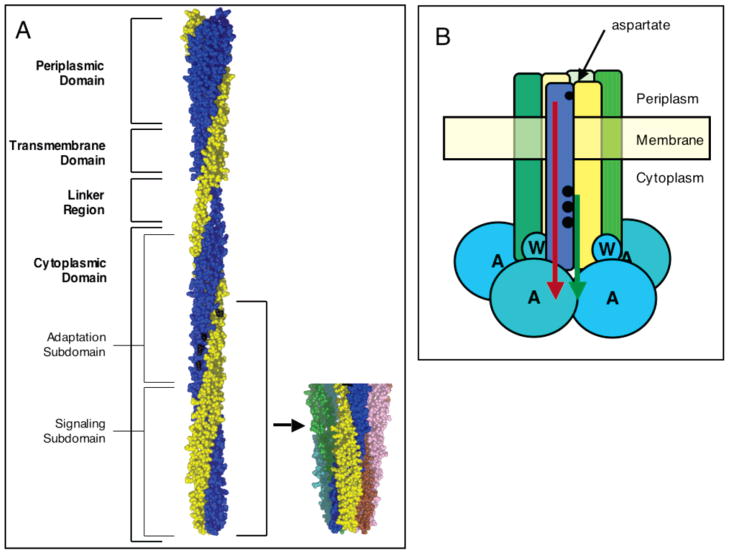
Figure 1.
Model of aspartate receptor structure and function. (A) Full-length model based on crystallographic and chemical data (18, 29, 37–39). Recent data support the model, except for the indicated helical conformation of the linker region (40, 41). The two identical subunits are colored blue and gold, respectively, and sites of methylation are colored black. Also shown is the crystal structure of the cytoplasmic domain fragment illustrating the trimer of dimers (5). Structures were generated with MacPymol (DeLano Scientific). (B) Schematic view of the core ternary signaling complex consisting of the receptor trimer of dimers, the coupling protein (CheW), and the histidine kinase (CheA). Accurate stoichiometries of the latter two components are not yet known. Two types of signals regulate kinase activity: the transmembrane attractant signal which inhibits the kinase (red) and the cytoplasmic adaptation signal which activates the kinase (green). These two signals originate at the periplasmic aspartate binding site (small black circle) and at the cytoplasmic adaptation sites (larger black circles), respectively.