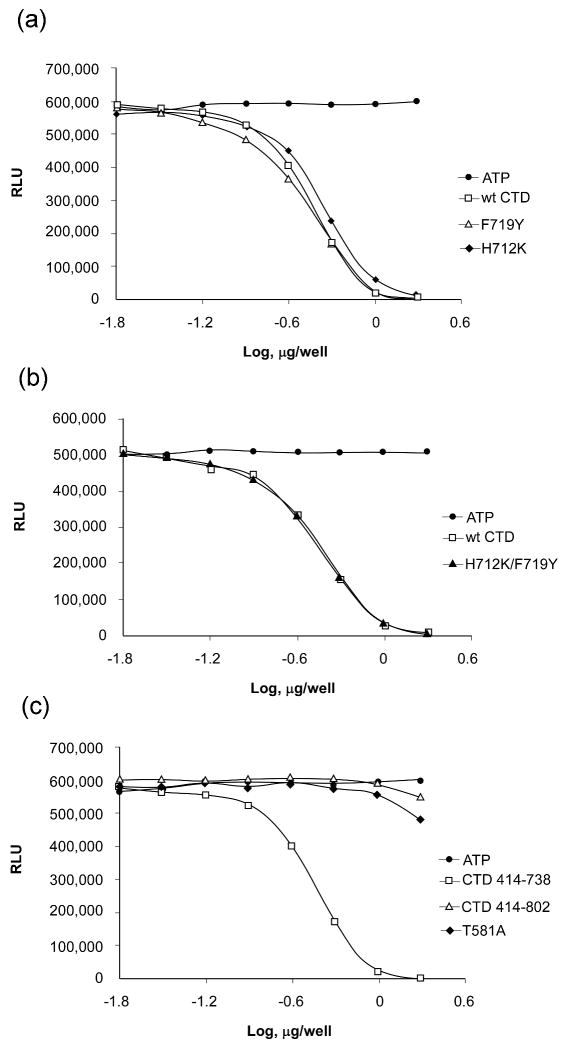
Figure 5. The kinase autophosphorylation assay for wildtype and CTD MSK1 mutants.
(A) The single point mutants, H712K and F712Y, exhibited autophosphorylation activity comparable to wildtype CTD MSK1. (B) The double mutant, H712K/F719Y, also had activity that was similar to the wildtype protein. (C) The CTD MSK1 (residues 414-738), which lacks the MAP kinase-docking site, had substantially higher activity compared with either the mutant T581A or the “full length” CTD (residues 414-802) protein. The longer CTD fragment and the T581A mutant had low residual activity and neither protein could complete the kinase reaction even up to 10 μg protein. Each experiment was repeated at least 3 times and data are expressed as Relative Luminescent Units (RLU).