Table 1.
Optimization of Alkynylation Conditionsa
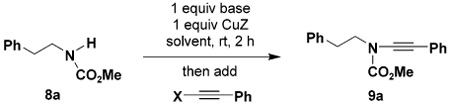 | |||||
|---|---|---|---|---|---|
| entry | base | CuZ | solvent | alkyne equiv, X |
yield (%)b |
| 1 | KHMDS | CuI | pyr | 1.0, I | 44 |
| 2 | KHMDS | CuI | pyr | 1.0, Br | 48 |
| 3 | KHMDS | CuI | pyrc | 1.0, Cl | 23 |
| 4 | KHMDS | CuI | DMF | 1.0, Br | 26 |
| 5 | n-BuLi | CuI | pyr | 1.0, Br | 0 |
| 6 | n-BuLi | CuI | DMSO | 1.0, Br | 20 |
| 7 | n-BuLi | CuTCd | DMSO | 1.0, Br | 24 |
| 8 | KHMDS | CuI | pyr | 0.6, Br | 40e |
| 9 | KHMDS | CuI | pyr | 2.0, Br | 67f |
| 10 | KHMDS | CuCN | pyr | 2.0, Br | 41 |
| 11 | KHMDS | CuI | tol, diamineg | 2.0, Br | 40 |
| 12 | KHMDS | CuI | pyr | 5.0, Br | 28h |
All reactions were carried out at rt for 20 h unless otherwise indicated. Concentration of 8a (0.200 g scale) was 0.15 M. Alkynes were added as benzene solutions.
Isolated yields of products purified by column chromatography.
Reaction at 75 °C.
Copper(I) thiophene-2-carboxylate.
Yield based on 1-bromo-2-phenylethyne.
Yield improved to 76% when scale increased to 2.0 g of 8a.
4.0 equiv of diamine ligand MeN(H)CH2-CH2N(H)Me.
Reaction for 90 h.
