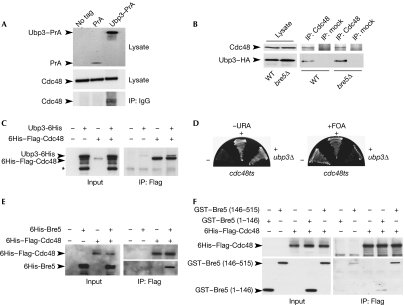
Figure 1.
Ubp3–Bre5 deubiquitination complex interacts physically and genetically with Cdc48. (A) Lysates from untagged cells or cells expressing protein A (PrA) or Ubp3–PrA were affinity purified on immunoglobulin G-coupled magnetic beads. Lysates or bound proteins were analysed by western blotting using Cdc48 antibodies (lower panels) or only secondary antibodies (upper panel). (B) Lysates from wild-type (WT) or bre5Δ cells expressing Ubp3–HA were immunoprecipitated (IP) using anti-Cdc48 or mock antibodies and analysed by western blotting with the indicated antibodies. Anti-Flag–agarose beads were used to immunoprecipitate purified recombinant 6His–Flag-Cdc48 (1 μg) in the absence or presence of recombinant (C) Ubp3–6His (3 μg), (E) 6His–Bre5 (1.2 μg) or (F) GST–Bre5 mutant proteins (1 μg). Anti-Flag–agarose beads were used as the control. Approximately, 3% of input or 50% of bound proteins were analysed by western blotting using anti-6His (C,E (upper panel), F (upper panel)), anti-Bre5 (E, lower panel), or anti-GST (F, lower panel). (*) indicates the major degradation product of Ubp3–6His. (D) The cdc48-6 thermosensitive mutant cells were transformed (+) or not (−) with p426-UBP3 (URA3) plasmid, before the deletion of the genomic copy of UBP3 gene (ubp3Δ). Transformants were streaked subsequently on 5-fluoroorotic acid (5-FOA) medium. Bre5, brefeldin A sensitivity 5; Cdc48, cell division cycle 48; GST, glutathione-Stransferase; HA, haemagglutinin; IP, immunoprecipitated; PrA, protein A; Ubp3, ubiquitin-specific processing protease 3; WT, wild type.