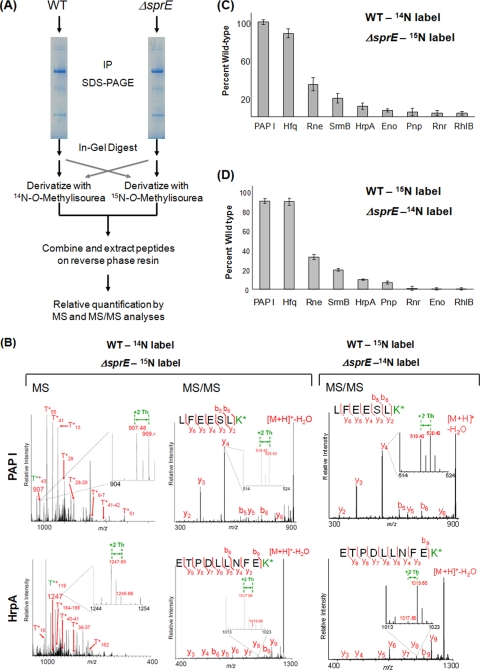
FIG. 3.
Relative quantification of PAP I's interacting partners using guanidination. (A) Workflow of the guanidination procedure, described in detail in Materials and Methods. After in-gel digestion with trypsin, the guanidination reaction was carried out using differentially labeled N-O-methylisourea. The wild-type peptides were labeled with 14N, which shifts the mass of lysine-containing peptides by +42 Th, and the ΔsprE peptides were labeled with 15N, for a shift of +44 Th. After the samples were mixed, relative quantification was performed by comparing the intensities of the light and heavy monoisotopic peaks. A parallel experiment was performed using reverse labeling. IP, immunopurification. (B) Sample spectra of guanidinated PAP I and HrpA at the MS and MS/MS level. On the MS panels, representative arginine-containing peptides are labeled. The T*+ designation represents the tryptic fragments containing modified lysine residues. The enlarged regions show the peptide of interest, with the +2-Th shift between heavy and light peaks. The insets in the MS/MS spectra illustrate the +2-Th shift between y ions. K* refers to homoarginine. (C) The relative quantification of PAP I interacting partners in the ΔsprE background. Since the +2-Th-shifted peak represents a mixture of the monoisotopic ΔsprE peptides and the third isotope of the wild-type peaks, all intensities of the heavy peptides were adjusted using the formula (ΔsprEobs − ISOexp) + [PAP IWTobs − (PAP IΔSprEobs − ISOexp)], where ΔsprEobs is the intensity of any peptide from the ΔsprE samples (obs, observed) and ISOexp is the predicted isotopic peak intensity (exp, expected). All intensities were adjusted to that of PAP I to correct for differences in starting material (see Fig. S3 in the supplemental material). (D) Results of the reverse labeling experiment, where wild-type peptides were derivatized with [15N]O-methylisourea and ΔsprE peptides with [14N]O-methylisourea. The isotopic correction described in Fig. S3 in the supplemental material was therefore not necessary. The intensities of the peaks from the wild-type peptides were set at 100%, and the intensities of the ΔsprE peptides are reported relative to that. All intensities were adjusted to that of PAP I to correct for differences in starting material. Error bars show standard deviations. WT, wild type.