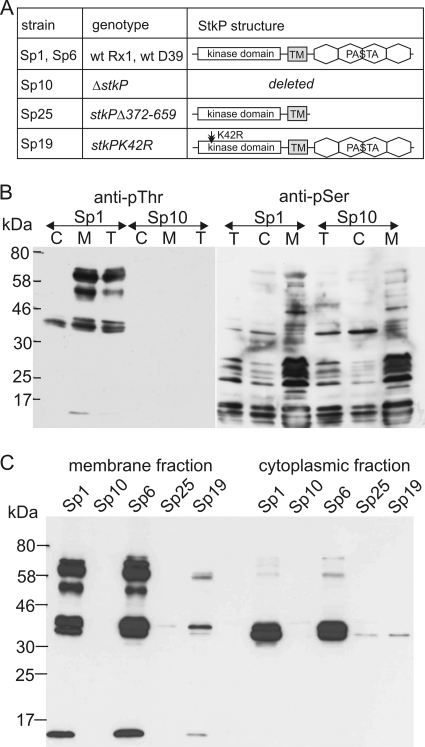
FIG. 1.
Detection of in vivo phosphorylated proteins in S. pneumoniae. wt, wild type; TM, transmembrane domain. (A) Pneumococcal strains used in this study and schematic diagrams of the StkP structure. (B) Phosphorylation of proteins on Thr is StkP dependent. Proteins phosphorylated on Thr (anti-pThr) and Ser (anti-pSer) were immunodetected in fractionated protein lysates of wild-type strain Sp1 and ΔstkP mutant strain Sp10. C, cytoplasmic fraction; M, membrane fraction; T, total protein lysate; anti-pThr, immunodetection with antibody against phosphothreonine; anti-pSer, immunodetection with antibody against phosphoserine. (C) Phosphorylation of proteins on Thr in S. pneumoniae depends on StkP catalytic activity. Proteins phosphorylated on Thr were immunodetected in membrane and cytoplasmic fractions of wild-type strains Sp1 (Rx1) and Sp6 (D39) and the following three stkP mutant strains: Sp10, a ΔstkP strain; Sp25, a strain expressing an isolated kinase domain anchored in the membrane; and Sp19, a strain expressing StkPK42R with reduced catalytic activity.