Abstract
Short- and long-term exposure to mild stress conditions can activate stress adaptation mechanisms in pathogens, resulting in a protective effect toward otherwise lethal stresses. The mesophilic strains Bacillus cereus ATCC 14579 and ATCC 10987 and the psychrotolerant strain B. weihenstephanensis KBAB4 were cultured at 12°C and 30°C until the exponential growth phase (i) in the absence of salt, (ii) in the presence of salt, and (iii) with salt shock after they reached the exponential growth phase and subsequently heat inactivated. Both the first-order model and the Weibull model were fitted to the inactivation kinetics, and statistical indices were calculated to select for each condition the most appropriate model to describe the inactivation data. The third-decimal reduction times (which reflected the times needed to reduce the initial number of microorganisms by three decimal powers) were determined for quantitative comparison. The heat resistance of both mesophilic strains increased when cells were salt cultured and salt shocked at 30°C, whereas these salt-induced effects were not significant for the psychrotolerant strain. In contrast, only the psychrotolerant strain showed salt-induced heat resistance when cells were cultured at 12°C. Therefore, culturing temperature and strain diversity are important aspects to address when adaptive stress responses are quantified. The activated adaptive stress response had an even larger impact on the number of surviving microorganisms when the stress factor (i.e., salt) was still present during inactivation. These factors should be considered when stress-integrated predictive models are developed that can be used in the food industry to balance and optimize processing conditions of minimally processed foods.
Bacillus cereus is a widespread, spore-forming pathogen that can be isolated from a range of different food products (4, 27), including pastry, vegetables and vegetable products, milk and milk products, and ready-to-eat foods. This toxin-producing pathogen can cause diarrhea and emesis (13, 25). The diarrheal syndrome is caused by several enterotoxins which are produced by vegetative cells in the small intestine. The emetic toxin, cereulide, causes emesis and is produced in foods before ingestion. Adequate chilling of foods is important to control the growth and toxin production of enterotoxin-producing (17) and emetic toxin-producing (7, 18) B. cereus strains.
During processing and storage of mildly processed foods, bacteria are exposed to one or more preservation stresses, known as hurdles (16). While individual hurdles might not be effective in controlling microbial growth, the right combination of hurdles can be powerful in controlling microbial growth in minimally processed foods. However, the potential of Bacillus to become more resistant to stresses challenges the effectiveness of minimal processing. Several studies have demonstrated that exposure to mild stressing conditions can result in the increased resistance of both mesophilic and psychrotolerant members of the B. cereus group (2, 3, 5, 21, 22). These studies used optimal culturing temperature during mild stress exposure to investigate the adaptive stress responses. However, during processing, distribution, and storage, the temperature of foods may be lower because chilling is commonly used in the minimal processing food chain. Therefore, investigation of the effect of low incubation temperature on the adaptive stress responses of food-borne bacteria is of great relevance and could provide valuable information for quantitative exposure assessment studies.
In the study described here, three representatives of the B. cereus group (12), namely, the mesophilic strains B. cereus ATCC 14579 and ATCC 10987 and the psychrotolerant strain Bacillus weihenstephanensis KBAB4, were cultured at 30°C in the absence and presence of mild salt stress, after which their heat resistance was assessed. Moreover, the culturing of cells was also performed at 12°C to determine the effect of a lowered culturing temperature on the adaptive salt stress responses. The third-decimal reduction time estimates were determined to evaluate the effects of the various culturing variables on the heat resistance of the three strains.
MATERIALS AND METHODS
Bacterial strain and culturing conditions.
The mesophilic strains B. cereus ATCC 14579 (8, 11) and ATCC 10987 (23) and the psychrotolerant strain B. weihenstephanensis KBAB4 (15) were used throughout this study. The cultures were stored frozen in brain heart infusion (BHI) broth (Becton Dickinson, France) supplemented with 25% (vol/vol) glycerol (Sigma, Netherlands) at −80°C. The bacteria were cultivated before each experiment in 10 ml BHI broth and incubated overnight at 30°C with shaking at 200 rpm (Innova 4335; New Brunswick Scientific, Netherlands). Afterwards, these cultures were inoculated in Erlenmeyer flasks (250 ml) containing 50 ml fresh BHI broth and incubated at 30°C (Julabo SW22; Julabo Labortechnik, Germany) and at 12°C (Forma orbital shaker 481; Thermo Electron Corporation) with shaking at 200 rpm until the stationary growth phase. The latter temperature was chosen because various isolates of the B. cereus group were unable to grow at 10°C (1).
Heat inactivation with and without preexposure to salt.
To investigate the effect of salt culturing and salt shock on subsequent heat resistance, the following procedure was followed. The stationary-phase cultures, which were precultured at 30°C, were inoculated in Erlenmeyer flasks containing 50 ml fresh BHI broth and BHI broth supplemented with 2.5% (wt/vol) sodium chloride (VWR, Belgium) and were further incubated at 30°C with shaking at 200 rpm, until the cells were exponentially growing (absorbance at 600 nm, 0.4 to 0.5; Novaspec II spectrophotometer; Pharmacia Biotech, United Kingdom). When they reached this optical density, both exponentially growing cultures, which were cultured in the absence of supplementary salt (untreated) or in the presence of supplementary salt (salt cultured), were further incubated for an extra period of 30 min at 30°C with shaking at 200 rpm. The concentration of 2.5% sodium chloride for salinity stressing was chosen because we previously demonstrated that short-term exposure of exponentially growing cells of B. cereus ATCC 14579 and ATCC 10987 to this salt concentration resulted in optimal salt-induced heat resistance for both strains (5). To expose exponentially growing cells to a salinity upshift for a short time interval rather than being cultured for a long-term interval in the presence of salt, the cells were cultured in BHI broth until the exponential growth phase, after which the BHI broth was supplemented with 2.5% (wt/vol) sodium chloride. These salt-shocked cultures were also incubated for 30 min at 30°C with shaking at 200 rpm. To investigate the effect of a lowered culturing temperature on the adaptive salt stress response, a similar procedure was followed to produce untreated, salt-cultured, and salt-shocked cultures at 12°C. The pH of the untreated, salt-cultured, and salt-shocked cultures of the three strains, grown at 30°C and 12°C, was measured and did not reach values below pH 7.0.
To heat inactivate the cells, the cultures were subsequently added (1:100, vol/vol) to 20 ml BHI broth and BHI broth supplemented with 2.5% (wt/vol) sodium chloride that were preheated to 50°C, 48°C, and 44.5°C for B. cereus ATCC 14579, B. cereus ATCC 10987, and B. weihenstephanensis KBAB4, respectively, and the cells were heat inactivated at the selected lethal temperatures with shaking at 200 rpm (Julabo SW22). Before and after heat exposure, samples were taken and decimal dilutions were made in peptone saline solution (1 g neutralized bacteriological peptone [Oxoid, United Kingdom] supplemented with 8.5 g sodium chloride per liter). The appropriate dilutions were surface plated, in duplicate, on BHI agar plates (BHI broth supplemented with 15 g agar [Oxoid] per liter) using a spiral plater (Eddy Jet; IUL Instruments, Spain). The plates were incubated at 30°C for 16 to 24 h, and the numbers of CFU were expressed in log10 CFU ml−1. For all experimental conditions, three independent reproductions were performed.
Microbial survival model fitting.
Two microbial survival models were fitted to the inactivation data of the three reproductions together for each experimental condition for the three strains using the program TableCurve 2D (Windows, version 2.03).
(i) The first-order model was as follows:
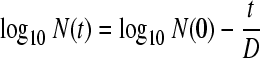 |
(1) |
where log10 N(t) is the log10 number of microorganisms at time t, log10 N(0) is the initial log10 number of microorganisms, and D is the time (in minutes) needed to reduce the number of microorganisms by one decimal.
(ii) The Weibull model was as follows:
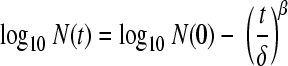 |
(2) |
where δ is the first-decimal reduction time (min) and β is a fitting parameter that defines the shape of the curve.
To evaluate the model-fitting performances of the first-order model and the Weibull model, a procedure similar to that described previously was followed (5). The following statistical indices were calculated.
(i) MSEmodel.
The lower the mean square error of the model (MSEmodel) is, the better the adequacy of the model to describe the data. It was calculated as follows:
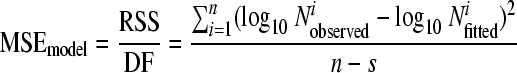 |
(3) |
where RSS is the residual sum of squares, DF is the degrees of freedom, n is the number of data points, s is the number of parameters of the model, log10 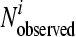 is the observed log10 number of microorganisms for data point i, and log10
is the observed log10 number of microorganisms for data point i, and log10 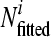 is the fitted log10 number of microorganisms for data point i.
is the fitted log10 number of microorganisms for data point i.
(ii) MSEdata.
The lower the mean square error of the data (MSEdata, which indicates the measuring error) is, the less variation was observed between the reproductions per experimental condition. It was calculated as follows:
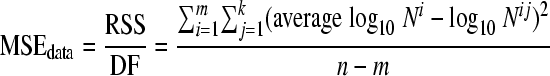 |
(4) |
where n is the number of data points, m is the number of sampling time points, k is the number of reproductions at each sampling time point, average log10 Ni is the mean value of the log10 number of microorganisms at sampling time point i, and log10 Nij is the log10 number of microorganisms at sampling time point i for specific reproduction j.
(iii) F test.
The F test was used to determine if the fitting performance of the model was statistically acceptable. The f value was calculated by the following equation:
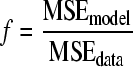 |
(5) |
The f value was tested against an F-table value (95% confidence). If the f value was smaller than the F-table value (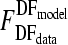 ), the F test was accepted and this indicated that the model fitting was statistically acceptable.
), the F test was accepted and this indicated that the model fitting was statistically acceptable.
For some experimental conditions, the fitting performances of both the first-order model and the Weibull model were statistically acceptable, according to the F test (equation 5). To evaluate whether the extra model parameter β of the Weibull model significantly improved the model description of these inactivation data sets, the 95% confidence interval of model parameter β was calculated and it was checked whether this confidence interval included the value of 1. Furthermore, an additional F test was performed (28):
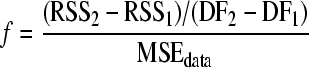 |
(6) |
where RSS1 is the residual sum of squares of the Weibull model, RSS2 is the residual sum of squares of the first-order model, and DF1 and DF2 are the degrees of freedom for the Weibull model and the first-order model, respectively. Note that DF2 − DF1 equals 1, because the difference between the number of model parameters of the Weibull model and the first-order model is 1. The f value was tested against an F-table value (95% confidence, 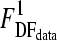 ). If the f value was smaller than the F-table value, the F test was accepted and this indicated that model parameter β did not significantly improve the description of the inactivation data.
). If the f value was smaller than the F-table value, the F test was accepted and this indicated that model parameter β did not significantly improve the description of the inactivation data.
Comparison of model parameters.
In order to compare the various conditions, the third-decimal reduction time, which reflected the time needed to reduce the initial number of microorganism by three decimal powers, was estimated for each condition. The third-decimal reduction time could be estimated without extrapolation outside the experimental ranges. For each condition, the selected model (first-order model or Weibull model) was fitted to the three independent reproductions individually, and the average third-decimal reduction time estimate was calculated. Independent t tests (two-sided) were performed to compare the average third-decimal reduction time estimates of the different conditions and to investigate if there were significant effects of short- and long-term salt stress exposure at 30°C and 12°C on subsequent heat resistance (SPSS, version 15.0.1, for Windows).
RESULTS
Effect of salt culturing and salt shock on subsequent heat resistance.
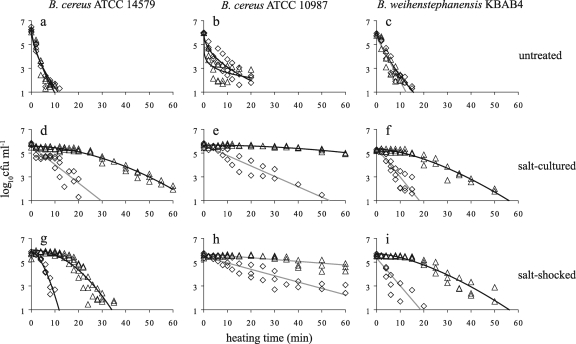
The mesophilic strains B. cereus ATCC 14579 and ATCC 10987 and the psychrotolerant strain B. weihenstephanensis KBAB4 were cultured until the exponential growth phase (i) in the absence of salt (untreated), (ii) in the presence of salt (salt cultured), and (iii) with salt shock for 30 min after they reached the exponential growth phase (salt shocked). Subsequently, the heat resistance of the untreated, salt-cultured, and salt-shocked cells was assessed. Figure 1 shows the inactivation kinetics of the cells that were cultured at 30°C. The temperatures selected to inactivate the three strains were not similar, namely, 50°C, 48°C, and 44.5°C for B. cereus ATCC 14579, B. cereus ATCC 10987, and B. weihenstephanensis KBAB4, respectively. These inactivation temperatures were chosen to obtain comparable inactivation kinetics for the untreated cultures of the three strains (Fig. 1a to c). The heat resistance of both mesophilic strains, B. cereus ATCC 14579 and ATCC 10987, increased when cells were salt cultured at 30°C (Fig. 1d and e) or preexposed to salt for 30 min at 30°C (Fig. 1g and h) compared to the heat resistance of the untreated cultures of both strains, whereas the effects of salt culturing or salt shock on heat resistance were less pronounced for the psychrotolerant strain, B. weihenstephanensis KBAB4 (Fig. 1f and i).
FIG. 1.
Heat inactivation kinetics of Bacillus cereus ATCC 14579 (a, d, and g), Bacillus cereus ATCC 10987 (b, e, and h), and Bacillus weihenstephanensis KBAB4 (c, f, and i). Cells were cultured at 30°C until the exponential growth phase in the absence of salt (a, b, and c), in the presence of salt (d, e, and f), or with salt shock for 30 min after they reached the exponential growth phase (g, h, and i) and were subsequently exposed to heat in the absence of salt (⋄) and in the presence of salt (▵). Continuous curves, fitting of the selected microbial survival model; black lines, fitting of the Weibull model; gray lines, fitting of the first-order model.
The addition of salt in the inactivation medium did not increase the heat resistance of cells that were not preexposed to salt (Fig. 1a to c) at 30°C. However, the presence of salt in the inactivation medium had a notable increasing protective effect for the salt-cultured and salt-shocked cells of all the three strains (Fig. 1d to i).
Effect of culturing temperature on salt-induced heat resistance.
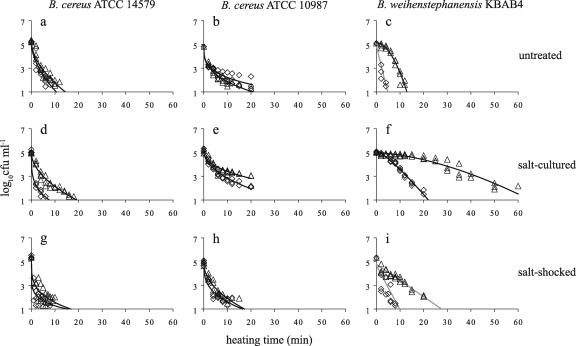
To study the effect of a lowered culturing temperature on the adaptive responses to salt stress, cells were also cultured at 12°C in the absence of salt (untreated) and in the presence of salt (salt cultured) or were salt shocked at 12°C for 30 min after they reached the exponential growth phase at 12°C (salt shocked) (Fig. 2). In contrast to culturing at 30°C, neither of the mesophilic strains showed increased heat resistance when cultured in the presence of salt at 12°C (Fig. 2d and e) or were salt shocked after they reached the exponential growth phase at 12°C (Fig. 2g and h) compared to the heat resistance of cells that were not preexposed to salt stress before heat inactivation (Fig. 2a and b). However, salt culturing (Fig. 2f) and, to a more limited extent, salt shock at 12°C (Fig. 2i) affected the heat resistance of the psychrotolerant strain, resulting in the increased heat resistance of this strain compared to that after culturing at 12°C without salt stress (Fig. 2c). The presence of salt in the inactivation medium increased the heat resistance of the untreated, salt-cultured, and salt-shocked cells of B. weihenstephanensis KBAB4 (Fig. 2c, f, and i).
FIG. 2.
Heat inactivation kinetics of Bacillus cereus ATCC 14579 (a, d, and g), Bacillus cereus ATCC 10987 (b, e, and h), and Bacillus weihenstephanensis KBAB4 (c, f, and i). Cells were cultured at 12°C until the exponential growth phase in the absence of salt (a, b, and c), in the presence of salt (d, e, and f), or with salt shock for 30 min after they reached the exponential growth phase (g, h, and i) and were subsequently exposed to heat in the absence of salt (⋄) and in the presence of salt (▵). Continuous curves, fitting of the selected microbial survival model; black lines, fitting of the Weibull model; gray lines, fitting of the first-order model.
Because species of the B. cereus group are spore formers, the potential presence of spores was determined in the untreated, salt-cultured, and salt-shocked cultures of the three strains grown at 30°C and 12°C. For that, the cultures were heated for 15 min at 75°C to kill the vegetative cells and to determine the number of spores. The number of spores in all the cultures was below the detection limit of 5 spores per ml, and therefore, spores could not have contributed to the observed inactivation curvature characteristics shown in Fig. 1 and 2.
Quantification of the effect of culturing temperature on salt-induced heat resistance.
In order to select the most appropriate model to quantitatively describe the heat inactivation kinetics of the three strains, the first-order model and the Weibull model were fitted to the replicate inactivation data together for each experimental condition. According to the F test, which statistically evaluated the mean square error of the model fitting compared to the measuring error (mean square error of the data) (equation 5), the fitting performance of the Weibull model was statistically acceptable for all the conditions (12 inactivation data sets per strain). For some experimental conditions, the performances of both the first-order model and the Weibull model were statistically acceptable, according to this F test. For these conditions, an additional F test (equation 6) was performed to evaluate whether the model parameter β of the Weibull model significantly improved the description of the inactivation data compared to that achieved with the first-order model. When the exclusion of model parameter β was statistically acceptable, the model parameter β was fixed equal to 1, resulting in the use of the first-order model to describe these inactivation data sets (10 out of 36 inactivation data sets). Under most of the conditions for which the exclusion of model parameter β was statistically acceptable, the confidence interval of model parameter β of the Weibull model also included 1, confirming an acceptable reduction of model complexity. For one experimental condition, the exclusion of model parameter β of the Weibull model, although it was statistically different from 1, was statistically acceptable according to the F test, indicating that in some boundary cases contradictory results can be obtained. The continuous curves in Fig. 1 and Fig. 2 show the fitting of the selected survival model (first-order model or Weibull model) for each experimental condition.
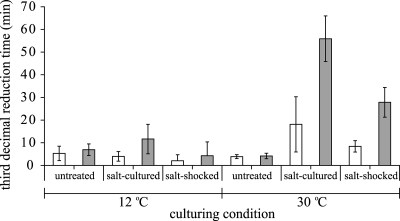
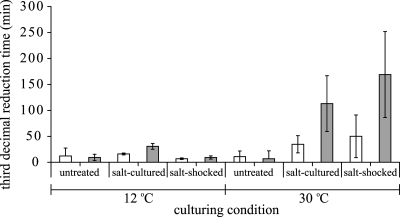
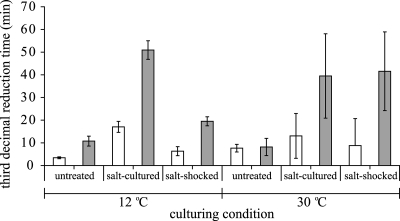
To quantify and evaluate the overall effects of the various culturing variables on heat resistance, the time to reach a 3-log10 reduction of the initial population was estimated for each experimental condition. For each experimental condition, the selected survival model was fitted to the individual reproductions, and the average estimate of the third-decimal reduction time was calculated. Figures 3 to 5 show the third-decimal reduction time estimates of the untreated, salt-cultured, and salt-shocked cells cultured at 12°C and 30°C of B. cereus ATCC 14579 (Fig. 3), B. cereus ATCC 10987 (Fig. 4), and B. weihenstephanensis KBAB4 (Fig. 5). The 95% confidence intervals of the third-decimal reduction time estimates clearly visualize the statistical comparison. Cells of the mesophilic strains B. cereus ATCC 14579 and ATCC 10987 that were salt cultured and salt shocked at 30°C were significantly more heat resistant than cells that were cultured at 30°C without salt stress (Fig. 3 and 4) (P < 0.05), and the third-decimal reduction time estimates of these salt-cultured and salt-shocked cells were two to five times higher than those of untreated cells. The statistical analyses confirmed that culturing temperature had a significant effect on the adaptive salt stress responses because salt culturing and salt shock did not significantly increase the level of heat resistance when both strains were cultured at 12°C (P > 0.05). The culturing temperature also significantly affected the adaptive salt stress response of psychrotolerant strain B. weihenstephanensis KBAB4 (Fig. 5), as salt culturing and salt shock increased the third-decimal reduction time estimates two to five times only when cells of B. weihenstephanensis KBAB4 were cultured at 12°C.
FIG. 3.
Third-decimal reduction time estimates of Bacillus cereus ATCC 14579 inactivated in the absence of salt (open bars) and in the presence of salt (gray bars). Before heat inactivation, the cells were cultured at 12°C and 30°C until the exponential growth phase in the absence of salt (untreated), in the presence of salt (salt cultured), or with salt shock for 30 min after they reached the exponential growth phase (salt shocked). Error bars indicate 95% confidence intervals.
FIG. 4.
Third-decimal reduction time estimates of Bacillus cereus ATCC 10987 inactivated in the absence of salt (open bars) and in the presence of salt (gray bars). Before heat inactivation, the cells were cultured at 12°C and 30°C until the exponential growth phase in the absence of salt (untreated), in the presence of salt (salt cultured), or with salt shock for 30 min after they reached the exponential growth phase (salt shocked). Error bars indicate 95% confidence intervals.
FIG. 5.
Third-decimal reduction time estimates of Bacillus weihenstephanensis KBAB4 inactivated in the absence of salt (open bars) and in the presence of salt (gray bars). Before heat inactivation, the cells were cultured at 12°C and 30°C until the exponential growth phase in the absence of salt (untreated), in the presence of salt (salt cultured), or with salt shock for 30 min after they reached the exponential growth phase (salt shocked). Error bars indicate 95% confidence intervals.
The parameter estimates presented in Fig. 3 to 5 can also be used to evaluate the impacts of the various culturing variables on heat resistance and distinguish main variables from side variables. Figures 3 to 5 unambiguously show that for the cultures in which the salt adaptive stress response was significantly activated, the heat resistance significantly increased when salt was also present during the inactivation treatment, resulting in very heat resistant cells for all the three strains. Moreover, although salt culturing and salt shock at 30°C did not significantly increase the heat resistance for B. weihenstephanensis KBAB4, the presence of salt during inactivation of these salt-preexposed cells also resulted in significantly more heat-resistant cells. The third-decimal reduction time estimates of these very heat-resistant cells of B. cereus ATCC 14579, B. cereus ATCC 10987, and B. weihenstephanensis KBAB4 were 5 to 15 times higher than those of cells that were not exposed to salt during culturing and subsequent heat inactivation.
DISCUSSION
For minimally processed foods, various hurdles are combined to control food quality and microbial safety. Quantification of food production and distribution processes contributes to the balance and optimization of the quality and safety of these foods. Previous studies demonstrated that the adaptive stress responses of food-borne pathogens can have a large impact on their resistance (see, e.g., references 2, 3, 5, 14, 22, and 24) and should therefore be addressed in quantitative assessments of the inactivation kinetics of pathogens in the food chain.
Heating is a widely used method for microbial inactivation, and a thorough literature survey showed that classical first-order inactivation kinetics are the exception rather than the rule (26). Also in the present study, for most of the conditions tested, the nonlinear Weibull model described the inactivation data significantly more adequately than the first-order model. However, when a reduction of model complexity was statistically acceptable, the first-order model was preferred to describe the inactivation data. This principle of parsimony, meaning that the model with the smallest number of parameters that adequately represents the data is preferred above a more complex model, is a strong model selection criterion. The biological variation influenced the most appropriate model selection because the higher the measuring error is, the simpler a model can be to adequately describe the data, and therefore reproductions of experiments should be performed to evaluate deviations from first-order kinetics are statistically relevant. In this study, the third-decimal reduction times were estimated to evaluate the effects of the various culturing variables on subsequent heat resistance. The third-decimal reduction time is an interpretable model parameter that could be easily recognized in the inactivation curvatures and reflected the time needed to produce a substantial inactivation of the pathogen without extrapolation outside the experimental ranges, and therefore, these estimates were used to evaluate the overall effects of culturing variables and to separate the main culturing variables from side culturing variables. Noteworthy are that the conclusions based on the third-decimal time estimates were rather comparable to those based on the first-decimal reduction time estimates (data not shown), confirming the observed trends.
The adaptive salt stress response depended on the culturing temperature, the stress exposure time (salt cultured versus salt shocked), and differences between the strains. Both salt culturing and salt shock at 30°C increased the subsequent heat resistance of mesophilic strains B. cereus ATCC 14579 and ATCC 10987, whereas these salt-induced effects were not significant for psychrotolerant strain B. weihenstephanensis KBAB4. Increased heat resistance after short-term preexposure to salt was previously observed for B. cereus ATCC 14579 (5, 22), B. cereus ATCC 10987 (5), B. cereus NCIMB 11796 (3), and B. weihenstephanensis DSM11827 (21). It was demonstrated that some proteins induced upon heat shock were also induced after salinity upshift, and this could have contributed to the salt-induced cross-protective effects toward heat. Moreover, complementary adaptation mechanisms can be involved, as genes encoding various osmoprotectant transporters were highly upregulated in B. cereus ATCC 14579 in response to increased osmolarity (6), and it was shown that osmoprotectants (e.g., glycine betaine) can serve as thermoprotectants (10). The extended use of chilling in the minimally processed food chain underlines the need to also investigate the adaptive stress responses of bacteria at nonoptimal incubation temperatures. As indicated by the limited available data describing the effect of low incubation temperature, the adaptive acid stress response of Listeria monocytogenes was reduced when the stressing temperature decreased (14). In the present study, we demonstrated that salt-induced thermotolerance was strongly influenced by the incubation temperature. Long- and short-term exposure to salt stress at 12°C did not significantly increase the heat resistance of either of the mesophilic strains. In contrast, psychrotolerant strain B. weihenstephanensis KBAB4 showed substantially increased heat resistance when cells were exposed to salt stress at 12°C, pointing to the importance of including differences between strains when adaptive stress responses are to be quantified.
The impact of an activated salt stress response on heat resistance can be larger when the stress factor is also present in the inactivation medium. The protective effect of salt in the inactivation medium was previously demonstrated for other pathogens, such as Salmonella enterica serovar Typhimurium (19), and tended to protect spores of B. cereus against heat (20). This may be explained by the increased stability of proteins (9). Our study showed that heat inactivation of salt-adapted cells in the presence of salt resulted in highly heat resistant cells of B. cereus ATCC 14579, B. cereus ATCC 10987, and B. weihenstephanensis KBAB4, and this can be of relevance for food-processing conditions when mild stress applications are subsequently followed by a heating processing step.
This study showed that culturing temperature and strain diversity are important aspects to address when adaptive stress responses are quantified and need to be considered in evaluations of adaptive stress responses. An activated adaptive response can have an even larger impact on the number of surviving organisms when the stress factor is still present during heat inactivation. These factors should be considered when stress-integrated predictive models are developed in order for the latter to be able to provide reliable predictions of the inactivation kinetics of microorganisms in minimally processed foods.
Acknowledgments
We thank Roy Moezelaar (Food and Biobased Research, Wageningen University and Research Centre, Wageningen, Netherlands) for valuable discussions and critical reading of the manuscript.
Footnotes
Published ahead of print on 7 May 2010.
REFERENCES
- 1.Afchain, A. L., F. Carlin, C. Nguyen-The, and I. Albert. 2008. Improving quantitative exposure assessment by considering genetic diversity of B. cereus in cooked, pasteurised and chilled foods. Int. J. Food Microbiol. 128:165-173. [DOI] [PubMed] [Google Scholar]
- 2.Browne, N., and B. C. A. Dowds. 2002. Acid stress in the food pathogen Bacillus cereus. J. Appl. Microbiol. 92:404-414. [DOI] [PubMed] [Google Scholar]
- 3.Browne, N., and B. C. A. Dowds. 2001. Heat and salt stress in the food pathogen Bacillus cereus. J. Appl. Microbiol. 91:1085-1094. [DOI] [PubMed] [Google Scholar]
- 4.Carlin, F., H. Girardin, M. W. Peck, S. C. Stringer, G. C. Barker, A. Martinez, A. Fernandez, P. Fernandez, W. M. Waites, S. Movahedi, F. van Leusden, M. Nauta, R. Moezelaar, M. Del Torre, and S. Litman. 2000. Research on factors allowing a risk assessment of spore-forming pathogenic bacteria in cooked chilled foods containing vegetables: a FAIR collaborative project. Int. J. Food Microbiol. 60:117-135. [DOI] [PubMed] [Google Scholar]
- 5.Den Besten, H. M. W., M. Mataragas, R. Moezelaar, T. Abee, and M. H. Zwietering. 2006. Quantification of the effects of salt stress and physiological state on thermotolerance of Bacillus cereus ATCC 10987 and ATCC 14579. Appl. Environ. Microbiol. 72:5884-5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Den Besten, H. M. W., M. Mols, R. Moezelaar, M. H. Zwietering, and T. Abee. 2009. Phenotypic and transcriptomic analyses of mildly and severely salt-stressed Bacillus cereus ATCC 14579 cells. Appl. Environ. Microbiol. 75:4111-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dierick, K., E. Van Coillie, I. Swiecicka, G. Meyfroidt, H. Devlieger, A. Meulemans, G. Hoedemaekers, L. Fourie, M. Heyndrickx, and J. Mahillon. 2005. Fatal family outbreak of Bacillus cereus-associated food poisoning. J. Clin. Microbiol. 43:4277-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankland, G. C., and P. F. Frankland. 1887. Studies on some new micro-organisms obtained from air. Philos. Trans. R. Soc. Lond. B Biol. Sci. 173:257-287. [Google Scholar]
- 9.Hansen, N.-H., and H. Riemann. 1963. Factors affecting the heat resistance of nonsporing organisms. J. Appl. Bacteriol. 26:314-333. [Google Scholar]
- 10.Holtmann, G., and E. Bremer. 2004. Thermoprotection of Bacillus subtilis by exogenously provided glycine betaine and structurally related compatible solutes: involvement of Opu transporters. J. Bacteriol. 186:1683-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanova, N., A. Sorokin, I. Anderson, N. Galleron, B. Candelon, V. Kapatral, A. Bhattacharyya, G. Reznik, N. Mikhailova, A. Lapidus, L. Chu, M. Mazur, E. Goltsman, N. Larsen, M. D'Souza, T. Walunas, Y. Grechkin, G. Pusch, R. Haselkorn, M. Fonstein, S. D. Ehrlich, R. Overbeek, and N. Kyrpides. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87-91. [DOI] [PubMed] [Google Scholar]
- 12.Kolstø, A.-B., N. J. Tourasse, and O. A. Økstad. 2009. What sets Bacillus anthracis apart from other Bacillus species? Annu. Rev. Microbiol. 63:451-476. [DOI] [PubMed] [Google Scholar]
- 13.Kotiranta, A., K. Lounatmaa, and M. Haapasalo. 2000. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2:189-198. [DOI] [PubMed] [Google Scholar]
- 14.Koutsoumanis, K. P., P. A. Kendall, and J. N. Sofos. 2003. Effect of food processing-related stresses on acid tolerance of Listeria monocytogenes. Appl. Environ. Microbiol. 69:7514-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapidus, A., E. Goltsman, S. Auger, N. Galleron, B. Ségurens, C. Dossat, M. L. Land, V. Broussolle, J. Brillard, M.-H. Guinebretiere, V. Sanchis, C. Nguen-The, D. Lereclus, P. Richardson, P. Wincker, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2008. Extending the Bacillus cereus group genomics to putative food-borne pathogens of different toxicity. Chem. Biol. Interact. 171:236-249. [DOI] [PubMed] [Google Scholar]
- 16.Leistner, L., and L. G. M. Gorris. 1995. Food preservation by hurdle technology. Trends Food Sci. Technol. 6:41-46. [Google Scholar]
- 17.Luby, S., J. Jones, H. Dowda, J. Kramer, and J. Horan. 1993. A large outbreak of gastroenteritis caused by diarrheal toxin-producing Bacillus cereus. J. Infect. Dis. 167:1452-1455. [DOI] [PubMed] [Google Scholar]
- 18.Mahler, H., A. Pasi, J. M. Kramer, P. Schulte, A. C. Scoging, W. Bär, and S. Krähenbühl. 1997. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N. Engl. J. Med. 336:1142-1148. [DOI] [PubMed] [Google Scholar]
- 19.Mañas, P., R. Pagan, I. Leguérinel, S. Condon, P. Mafart, and F. Sala. 2001. Effect of sodium chloride concentration on the heat resistance and recovery of Salmonella typhimurium. Int. J. Food Microbiol. 63:209-216. [DOI] [PubMed] [Google Scholar]
- 20.Mazas, M., S. Martínez, M. López, A. B. Alvarez, and R. Martin. 1999. Thermal inactivation of Bacillus cereus spores affected by the solutes used to control water activity of the heating medium. Int. J. Food Microbiol. 53:61-67. [DOI] [PubMed] [Google Scholar]
- 21.Periago, P. M., T. Abee, and J. A. Wouters. 2002. Analysis of the heat-adaptive response of psychrotrophic Bacillus weihenstephanensis. Int. J. Food Microbiol. 79:17-26. [DOI] [PubMed] [Google Scholar]
- 22.Periago, P. M., W. van Schaik, T. Abee, and J. A. Wouters. 2002. Identification of proteins involved in the heat stress response of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 68:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasko, D. A., J. Ravel, O. A. Økstad, E. Helgason, R. Z. Cer, L. Jiang, K. A. Shores, D. E. Fouts, N. J. Tourasse, S. V. Angiuoli, J. Kolonay, W. C. Nelson, A.-B. Kolstø, C. M. Fraser, and T. D. Read. 2004. The genome sequence of Bacillus cereus ATCC 10987 reveals metabolic adaptations and a large plasmid related to Bacillus anthracis pXO1. Nucleic Acids Res. 32:977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skandamis, P. N., Y. Yoon, J. D. Stopforth, P. A. Kendall, and J. N. Sofos. 2008. Heat and acid tolerance of Listeria monocytogenes after exposure to single and multiple sublethal stresses. Food Microbiol. 25:294-303. [DOI] [PubMed] [Google Scholar]
- 25.Stenfors Arnesen, L. P., A. Fagerlund, and P. E. Granum. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579-606. [DOI] [PubMed] [Google Scholar]
- 26.Van Boekel, M. A. J. S. 2002. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int. J. Food Microbiol. 74:139-159. [DOI] [PubMed] [Google Scholar]
- 27.Wijnands, L. M., J. B. Dufrenne, F. M. Rombouts, P. H. in't Veld, and F. M. van Leusden. 2006. Prevalence of potentially pathogenic Bacillus cereus in food commodities in The Netherlands. J. Food Prot. 69:2587-2594. [DOI] [PubMed] [Google Scholar]
- 28.Zwietering, M. H., J. T. de Koos, B. E. Hasenack, J. C. de Wit, and K. van't Riet. 1991. Modeling of bacterial growth as a function of temperature. Appl. Environ. Microbiol. 57:1094-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]