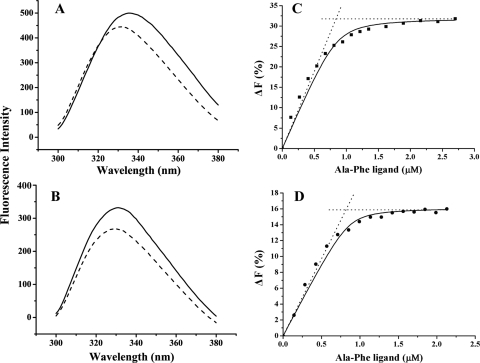
FIG. 3.
Intrinsic fluorescence change of PsDppA (A) and EcDppA (B) induced by the addition of saturating dipeptide Ala-Phe and fluorescence titration of PsDppA at 15°C (C) and EcDppA at 35°C (D). In an intrinsic fluorescence change assay (left), the emission spectra of DppA proteins with a concentration of ∼100 μg/ml in the absence (solid lines) or presence (broken lines) of saturating Ala-Phe were recorded at 15°C. In fluorescence titration (right), the concentration of DppA proteins was 0.86 μM. The solid line represents the best fitting of the data to equation 2 in Materials and Methods. The intersection point of the dotted lines corresponds to the binding stoichiometry. All of the experiments were performed in 25 mM phosphate buffer (pH 7.5). Each experiment was repeated five times, and similar results were obtained each time.