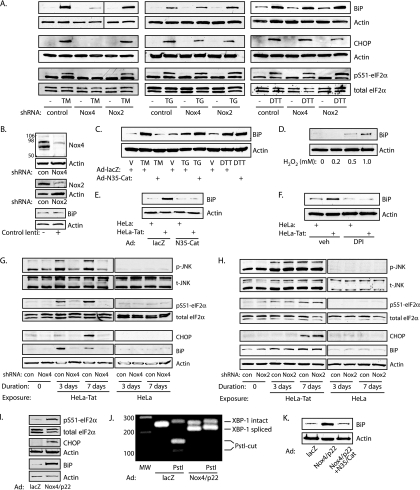
FIG. 4.
Nox4 mediates tunicamycin and Tat-induced UPR. (A) Lentiviral knockdown of Nox4 or Nox2 preceded exposure of HUVEC to tunicamycin (TM) (10 μg/ml), thapsigargin (TG) (0.4 μM), or DTT (2 mM) for 16 h. (B) Efficacy of knockdown. con, control; lenti, lentivirus. Numbers at the left indicate molecular mass markers in kilodaltons. (C) HUVEC were transduced with control (lacZ) or N35-Cat adenovirus and then exposed to vehicle (0.001% DMSO) (V), TM, TG, or DTT for 16 h. (D) HUVEC were exposed to the indicated concentrations of H2O2 for 16 h, and BiP was assessed by immunoblotting. (E) Cells were transduced with Ad-lacZ or Ad-N35-Cat and cocultured with HeLa or HeLa-Tat cells for 3 days. BiP expression is shown. (F) Cells were cocultured with HeLa or HeLa-Tat cells in the presence of vehicle (0.001% DMSO) or diphenylene iodonium (DPI) (10 μM) for 3 days, and BiP was assessed. (G) Following knockdown of Nox4, untreated HUVEC (lanes 1 and 2) or HUVEC cocultured with HeLa-Tat (lanes 3 to 6) or HeLa (lanes 7 to 10) cells for 3 or 7 days were immunoblotted as indicated. Knockdown of Nox4 completely blocked phosphorylation of JNK and eIF2α and induction of CHOP and BiP by HeLa-Tat cells. UPR markers were uninduced in HeLa-exposed cells. (H) Conditions were similar to those in panel G except following Nox2 instead of Nox4 knockdown. Nox2 knockdown had no effect on activation of the UPR. (I) Overexpression of Nox4 and p22phox by adenoviral transduction increased eIF2α phosphorylation and CHOP and BiP induction compared to Ad-lacZ infection. The blots are representative of 2 to 4 experiments. (J) RT-PCR of XBP-1 transcript demonstrating splicing of XBP-1 3 days after infection with Ad-Nox4/Ad-p22. (K) Nox4 and p22phox were overexpressed in the presence or absence of N35-Cat, as indicated, and the cells were immunoblotted for BiP.