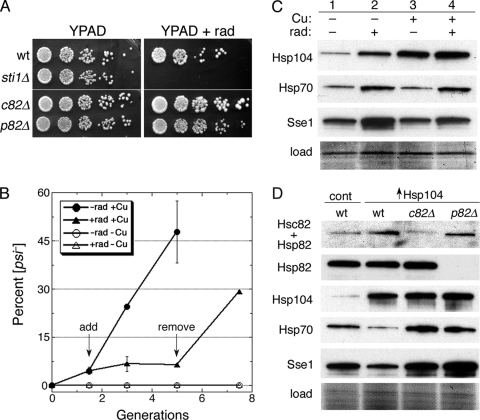
FIG. 5.
Hsp90 function is important for curing of [PSI+] by overexpressed Hsp104. (A) Sensitivity of strains (all are [PSI+]) to the Hsp90-specific inhibitor radicicol. Overnight YPAD cultures were diluted to an OD600 of 1.0, and 5-fold serial dilutions were spotted onto plates without and with (+rad) 25 μg/ml radicicol. Plates were scanned after incubation for 3 days at 30°C. (B) Radicicol blocks curing of [PSI+] by overexpressed Hsp104. Wild-type cells with plasmid for copper-inducible overexpression of Hsp104 were grown under inducing conditions (100 μM CuSO4) for 1.5 generations, at which time (“add” arrow) the overexpressing culture was split. One culture (closed circles) was untreated, while radicicol (25 μg/ml final concentration) was added to the other (closed triangles). After growing for another 3.5 generations (“remove” arrow), the radicicol-treated culture was washed, suspended in fresh radicicol-free medium, and grown for another 2.5 generations. Cultures grown without CuSO4 and either lacking (open circles) or containing (open triangles) radicicol were monitored in parallel. Values are averages of the results for three independent experiments ± standard deviation. (C) Western analysis of chaperones (indicated on left) in cells used in a replicate of the experiment shown in panel B. The “load” image shows a portion of the blotted membrane stained by amido black. (D) Western analysis of Hsp90 and other chaperones in hsc82Δ and hsp82Δ cells used in curing experiments. The blot in the top image is probed with an antibody that recognizes both isoforms of Hsp90 (Hsc82 + Hsp82), while the panel beneath it is an identical blot probed with a different antibody reactive only to the inducible Hsp82 isoform (see also Fig. 3). The lower panels show blots probed for Hsp104, Hsp70, and Sse1, as indicated. All cultures contained 100 μM CuSO4; control cells (cont) carry the empty vector.