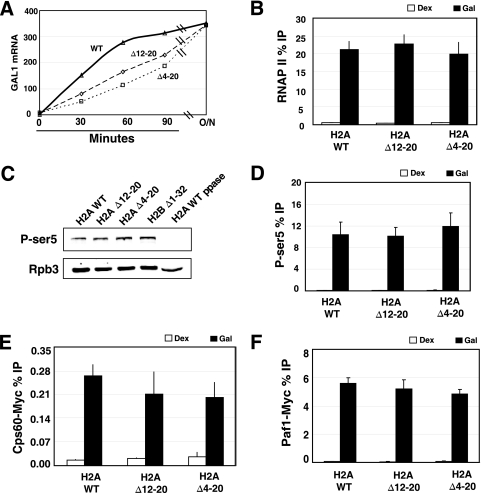
FIG. 3.
Examination of transcription factor recruitment to GAL1 in H2A mutants. (A) Northern blot of GAL1 mRNA. Wild-type, H2A Δ12-20, or H2A Δ4-20 strains were grown to log phase in medium containing 2% raffinose and induced with 2% galactose for the times indicated in the figure. For the overnight induction (O/N), cells were grown to log phase in medium containing 2% galactose. The levels of RNA were normalized to the signal of scR1, a loading control. (B) RNAPII cross-linking to the 5′ end of GAL1. 8WG16 was used to immunoprecipitate chromatin in cells grown in dextrose (Dex) or galactose (Gal). ChIP was performed as described in the legend to Fig. 2. Cells were grown in galactose for 16 h. (C) Western blot analysis of RNAPII Ser5 phosphorylation levels in whole-cell extracts. As a control for the selectivity of the antibody, extract from the wild-type cells was treated with lambda phosphatase (WT ppase) in lane 5. The extracts were also probed with antibody against Rpb3 to control for the amount of RNAPII. (D) The experiment was the same as that for panel B except for measurement of the cross-linking of Ser5-phosphorylated RNAPII (H14 antibody) over GAL1. (E and F) ChIP analysis of Cps60-Myc (E) and Paf1-Myc (F) recruitment to the 5′ end of GAL1.