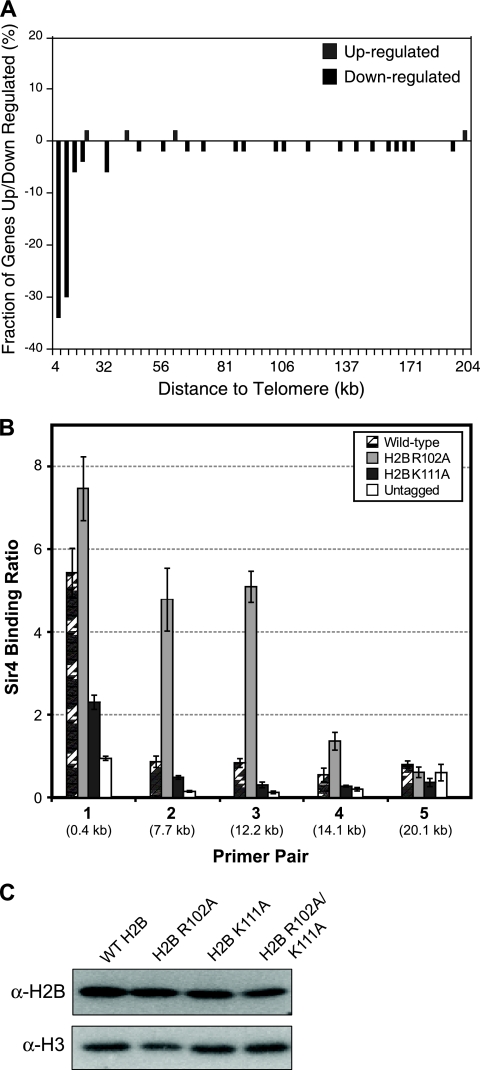
FIG. 5.
(A) The histone H2B R102A mutant represses the expression of telomere-proximal genes. Depicted is the chromosome plot of the genome-wide expression changes for the histone H2B R102A mutant relative to the wild type. A histogram of the fraction of genes whose mRNA levels are up- or downregulated is plotted as a function of their distance from a chromosome end. (B) The histone H2B R102A mutation causes spreading of Sir4 beyond the telomeres. ChIP analysis was used to examine the level of Sir4 protein binding to five regions on telomere V-L in wild-type and histone H2B R102A and K111A mutant strains. An untagged strain was used as a control, and Sir4 binding ratios were normalized to ACT1 using the input DNA, as previously described (21). Error bars represent the standard deviations from three independent samples. (C) Histone H2B core mutations do not alter the stability of the histone H2B protein or its incorporation into chromatin. Chromatin extracts from wild-type (WT) histone H2B and the R102A, K111A, and R102A/K111A mutants were prepared using a cell fractionation protocol previously described (23) and examined by Western blotting. The level of histone H2B protein was not significantly changed in the histone mutant strains compared to that in the wild type. Histone H3 was used as a loading control. α, anti.