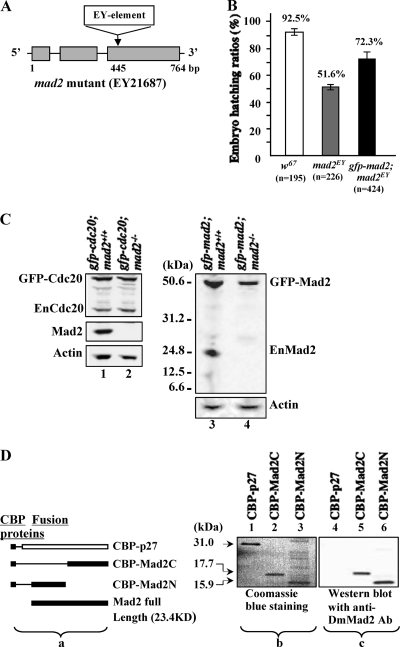
FIG. 1.
Characterization of the mad2EY mutant. (A) Schematic drawing showing the mad2EY mutant caused by an EY element insertion into the third exon at bp 445 of the mad2 gene region (4). (B) The mad2EY mutant can be maintained as laboratory homozygous stock with approximately 50%-reduced viability. This reduced-viability phenotype can be partially rescued by introducing an ectopically expressed transgene of gfp-mad2 (TM2-20) or mad2-Ypet (TM2-27; data not shown) on the second chromosome. The error bars indicate standard deviations. (C) Western blot results. (Lanes 1 and 2) GFP-Cdc20 protein in w67 and mad2EY-null mutant syncytial embryos, respectively. GFP-Cdc20 expression was around 2-fold that of endogenous Cdc20. (Lane 3) GFP-Mad2 and endogenous Mad2 expression in w67 embryos. Lanes 2 and 4 show no detectable endogenous Mad2, or any of its truncated forms, from mad2EY mutant embryo samples probed with an affinity-purified anti-full-length-DmMad2 antibody. (Lane 4) GFP-Mad2 was expressed at a level equivalent to that of the endogenous Mad2 in control samples (lane 3). Actin bands acted as loading controls. (D) (a) Schematic drawing showing the CBP-tagged fusion proteins used to test the anti-DmMad2 antibody. (b) Coomassie blue-stained 10% SDS-PAGE gel showing three purified fusion proteins, with molecule sizes indicated. The sample loading was as follows: lane 1, CBP-p27 (∼0.014 mg); lane 2, CBP-Mad2C (∼0.012 mg); lane 3, CBP-Mad2N (∼0.016 mg). (c) Western blot results showing that anti-DmMad2 antibody (Ab) can detect both the N- and C-terminal halves of truncated Mad2 fusions but not CBP-p27. Lane 4, CBP-p27; lane 5, CBP-Mad2C; lane 6, CBP-Mad2N. The samples loaded were 10 times less than those loaded for Coomassie blue staining. CBP-p27 (CBP plus full-length p27 protein) was used as the control to rule out nonspecific CBP signals.