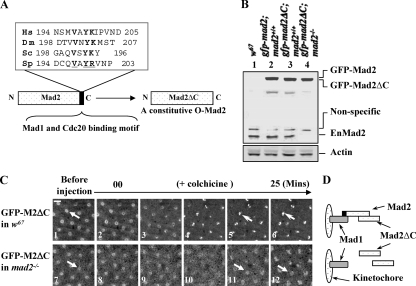
FIG. 8.
C-Mad2 and O-Mad2 can form heterodimers on kinetochores in living syncytial embryos, and O-Mad2 kinetochore localization is essential for Mad2-dependent SAC function. (A) Schematic drawing and sequence alignments showing the last 10 C-terminal amino acids of Mad2 from different species; the conserved residues are underlined and shown in boldface. The DmMad2ΔC construct with the deletion of the last 10 residues is illustrated. (B) Western blot results showing GFP-Mad2 and GFP-Mad2ΔC expression levels in syncytial embryos compared with endogenous Mad2 levels. Lane 1, w67 sample; lane 2, GFP-Mad2 and endogenous Mad2 levels in w67 embryo; lane 3, GFP-Mad2ΔC and endogenous Mad2 levels in w67 embryo; lane 4, GFP-Mad2ΔC level in mad2EY-null mutant embryo. Actin bands acted as loading controls. (C) GFP-Mad2ΔC can localize to kinetochores (top row, open arrows) in w67 embryos when endogenous Mad2 was present (top row, closed arrow indicates a nucleus before colchicine treatment) but not in a mad2EY-null mutant embryo (bottom row, closed arrows); both living embryos were treated with colchicine as described in Materials and Methods. Bar = 10 mm. (D) Schematic drawing showing the likely kinetochore behavior of GFP-Mad2ΔC in both scenarios.