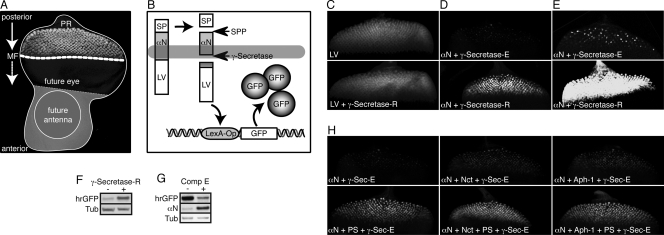
FIG. 4.
Visualization of enhanced γ-secretase activity in vivo. (A) Assembled confocal image of a Drosophila eye-antenna imaginal disc isolated from a late-third-instar larva. The disc was labeled with an antibody against the neuronal marker Elav, mainly localizing to the nucleus of photoreceptor neurons (PR) within the developing retina. In this larval stage, a morphogenetic furrow (MF) moves from posterior to anterior within the presumptive eye epithelium. Behind this furrow, and thus in reciprocal direction to the movement of the MF, the differentiation of photoreceptor clusters occurs, resulting in columns containing progressively more mature groups of differentiating cells. The GMR enhancer drives expression of GAL4 in all cells posterior to the morphogenetic furrow. (B) Schematic representation of the reporter system that permits the in vivo detection of intramembrane proteolysis at the cellular level. Expression of an engineered optimal Notch-based γ-secretase substrate (αN) is induced with GMR-GAL4. This type I transmembrane protein contains a truncated extracellular domain and is fused at its intracellular C terminus to the synthetic transcription factor LexA-VP16 (LV). Thus, cleavage by the signal peptide peptidase (SPP) mimics extracellular shedding and induces the concomitant release of a membrane-anchored CTF. This CTF is recognized by γ-secretase as a substrate, which results in its intramembrane proteolysis and the release of the intracellular fragment ICD. The synthetic transcription factor LV domain fused to the ICD then translocates to the nucleus, resulting in its binding to the LexA-Operator sequence present in the transgenic reporter construct. As a consequence, GFP expression is induced specifically in only those cells with recent and/or ongoing γ-secretase activity. (C) Maximum projections of confocal sections of eye imaginal discs expressing γ-secretase-R together with the reporter system to detect the activity of soluble LV. Depicted is the output of the reporter system (GFP fluorescence) in the absence (top) and presence (bottom) of γ-secretase-R, and no effect of γ-secretase-R on LV activity could be observed. The images were recorded simultaneously under identical conditions. (D) Maximum projections of confocal sections of eye imaginal discs expressing γ-secretase-R together with the reporter system to detect the intramembrane cleavage of αN. Depicted is the output of this cleavage-dependent reporter system (GFP fluorescence) in the absence (top) and presence (bottom) of γ-secretase-R. The images were recorded simultaneously under identical conditions. (E) Experiment similar to that in panel D, but the imaging conditions were adjusted to allow enhanced visualization of the signal generated by γ-secretase-E. (F) αN reporter larvae possessing γ-secretase-E but lacking γ-secretase-R (−) and αN reporter larvae expressing γ-secretase-R (+) were allowed to develop to adults, and their adult heads were subjected to protein immunoblot analyses to visualize changes in GFP expression (hrGFP) indicative of overall γ-secretase proteolytic activity. Tub, tubulin loading control. (G) Protein immunoblots from eye imaginal discs expressing the αN reporter system alone, which were cultured in vitro in the absence or presence of the γ-secretase inhibitor compound E (Comp E). Inhibition of γ-secretase-E resulted in an increase in the amount of uncleaved substrate and a concomitant decrease in GFP expression. (H) Maximum projections of confocal sections of eye imaginal discs from transgenic larvae in which different combinations of individual γ-secretase-R components were coexpressed in conjunction with the αN cleavage reporter system, as indicated at the bottom left of each image. Depicted is the output of the reporter system (GFP fluorescence) in each genotype. The images were recorded under identical conditions.