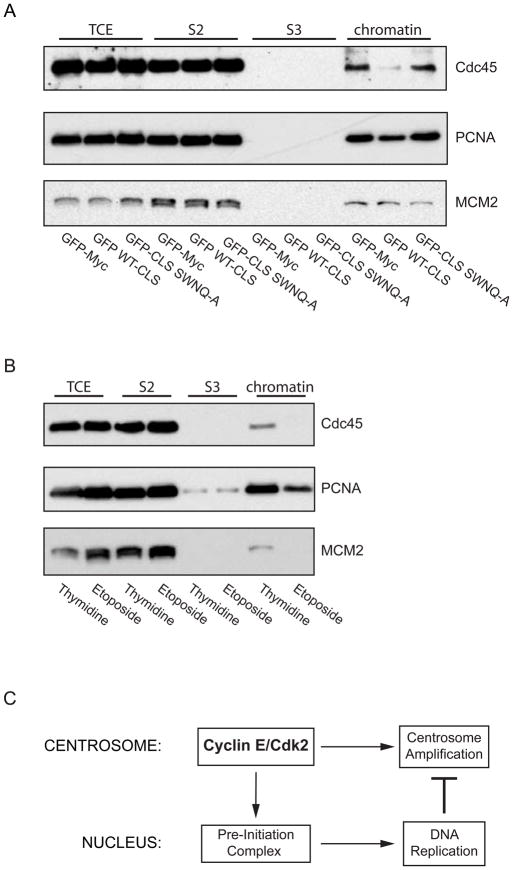
Figure 4. Expression of the cyclin E CLS prevents chromatin loading of pre-initiation complex proteins.
(A) Western blots of chromatin fractions from asynchronous CHO-K1 cells for Cdc45, PCNA, and MCM2 (loading control) in cells expressing GFP-Myc (control), the wild-type cyclin E CLS, or the mutant SWNQ-A CLS. The proteins from the total cell extract (TCE), in the soluble fraction (S2), the solubilized chromatin supernatant fraction (S3), and the chromatin-bound fraction (chromatin) are shown.
(B) Western blots of chromatin fractions as in Panel A from CHO-K1 cells synchronized at the G1-S boundary by double thymidine treatment or in G2 phase by etoposide treatment. The distribution of proteins from the total cell extract (TCE), in the soluble fraction (S2), solubilized nuclear protein fraction (S3) and chromatin-bound fraction (chromatin) are shown.
(C) A model for communication between the centrosome and the nucleus at the G1-S transition. The model depicts cyclin E-Cdk2 localization on the centrosome as contributing to Pre-Initiation complex formation on chromatin in the nucleus and to centrosome amplification. Once DNA synthesis commences, replication factors released from chromatin are recruited to centrosomes and inhibit over-duplication of centrosomes.