FIGURE 2.
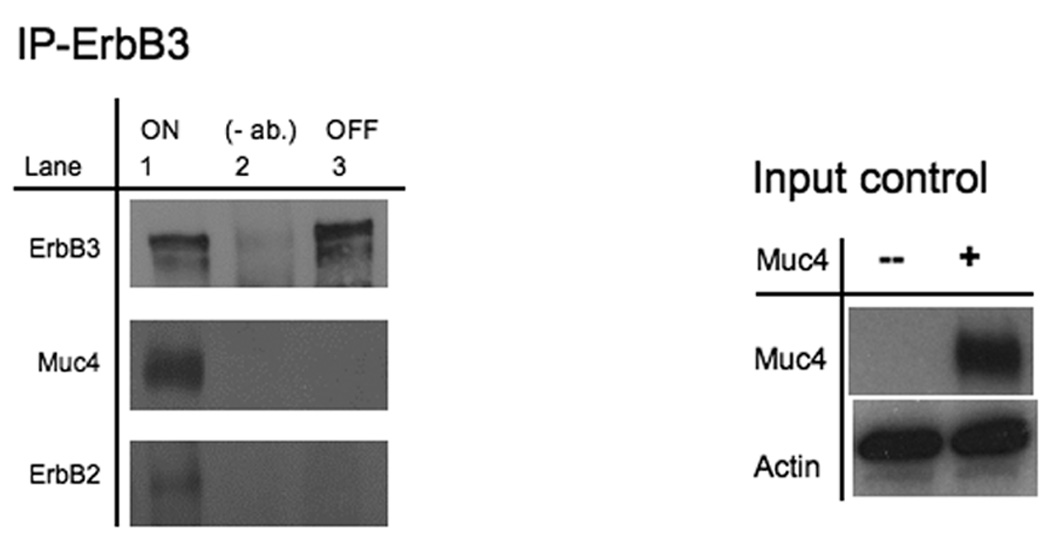
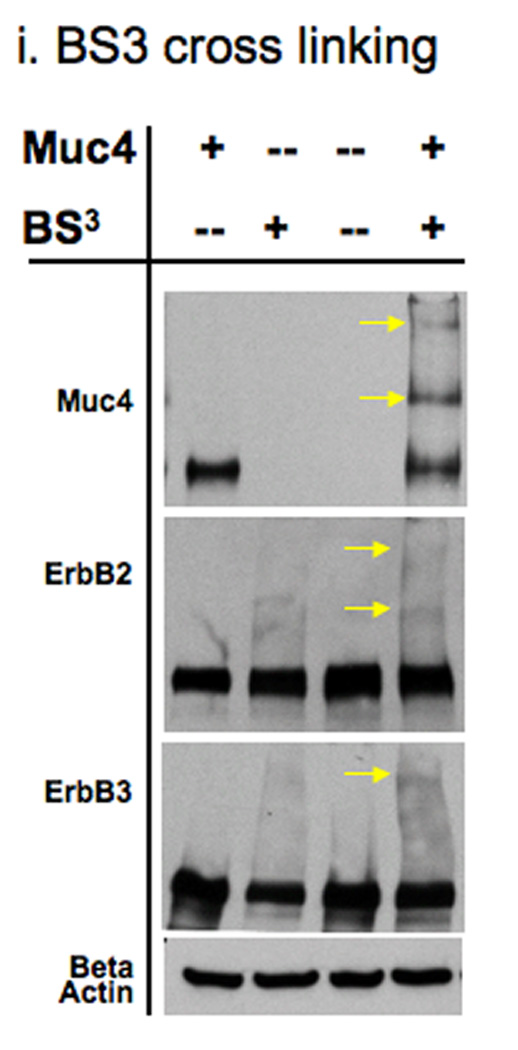
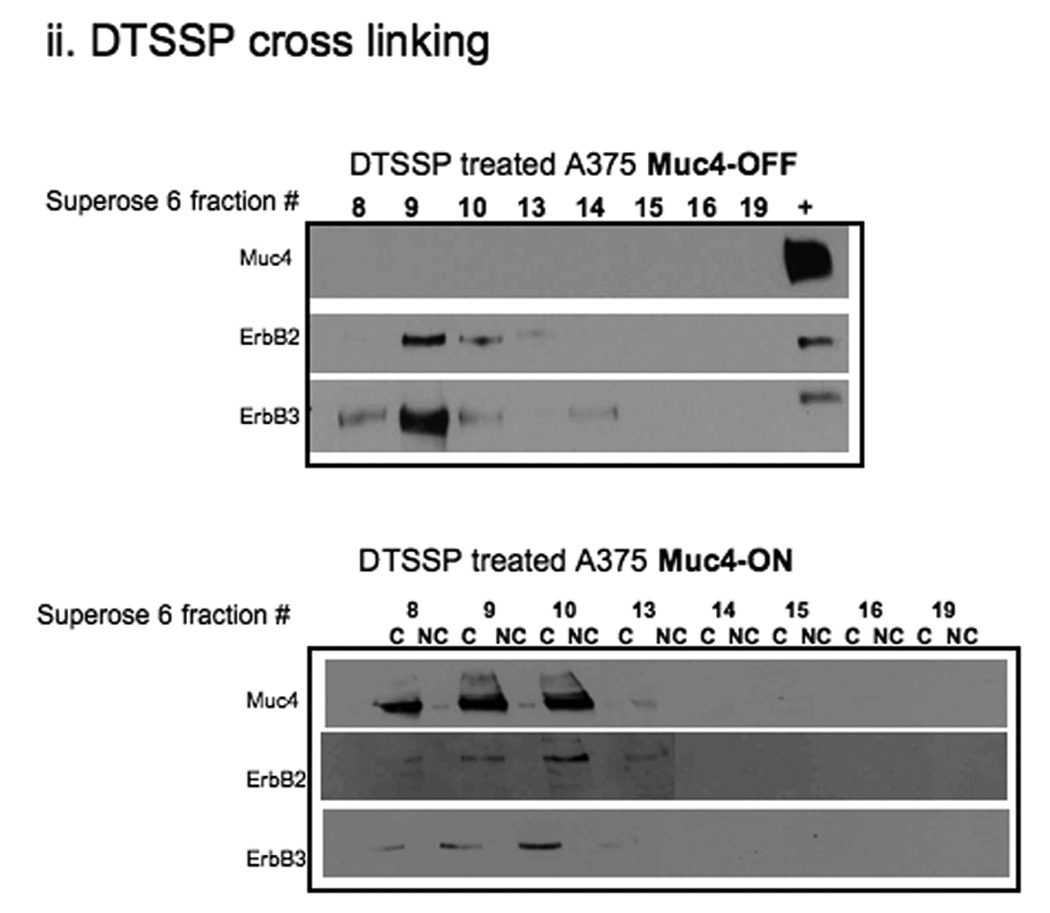
Muc4 modulates ErbB2 signaling potential by stabilizing and directly interacting with the ErbB2-ErbB3 heterodimer. A. Muc4 promotes stable ErbB2-ErbB3 interaction. Proteins from RIPA extracts of A375 cells with or without Muc4 (48 h) were subjected to immunoprecipitation with anti-ErbB3 (C-17) antibody. Immunoprecipitates were immunoblotted with anti-ErbB3, anti-Muc4, and anti-ErbB2 antibodies. The input control is an immunoblot of the lysates with anti-Muc4 and anti β-actin antibodies. B. Muc4 expression results in Muc4-ErbB2-ErbB3 complexes. Cellular chemical cross-linking immunoblots. A375 Muc4-transfected (Rep 3 clone) cells with or without Muc4 (48 h) were crossed linked with chemical cross-linking agents. i). With BS3 [1 mM] for 2 h on ice, and quenched with Tris-HCl [20–50 mM], pH 7.5 at room temperature for 15 min. Yellow arrows indicate cross-linked products. Cell lysates were separated on 4–15% gradient gels, and blotted with anti-Muc4, anti-ErbB2, anti-ErbB3, and β-actin antibodies. ii). With the reversible chemical cross linking analogue, DTSSP, at [1 mM] for 2 h, quenched with Tris-HCl [20–50 mM], pH 7.5 at room temperature for 15 min. Cross-linked cells were lysed in RIPA buffer without phosphatase inhibitors or dithiothreitol. Cleared lystes were loaded by FPLC (1 mL/min) onto a high resolution Superose 6 analytical gel filtration column equilibrated at 4°C in RIPA buffer without Triton X-100, inhibitors or dithiothreitol. Collected fractions were TCA-precipitated and separated on 4–15% gradient gels. Muc4-Off fractions were treated with 5% β-mercaptoethanol-containing SDS-PAGE sample buffer (top). Muc4-On fractions (bottom) were loaded side by side in duplicates with native SDS-PAGE sample buffer to represent non-cleaved (NC) samples, or with 5% β-mercaptoethanol containing SDS-PAGE sample buffer to examine the cleaved complex components (C). Samples were then immunoblotted with the indicated antibodies.
